Anat Cell Biol.
2024 Jun;57(2):278-287. 10.5115/acb.24.048.
Insertions of the striated muscles in the skin and mucosa: a histological study of fetuses and cadavers
- Affiliations
-
- 1Department of Anatomy, Jeonbuk National University Medical School, Jeonju, Korea
- 2Division of Internal Medicine, Iwamizawa Aska Hospital, Iwamizawa, Japan
- 3Department of Anatomy, Tokyo Dental College, Tokyo, Japan
- 4Department of Anatomy and Embryology, School of Medicine, Complutense University, Madrid, Spain
- KMID: 2556572
- DOI: http://doi.org/10.5115/acb.24.048
Abstract
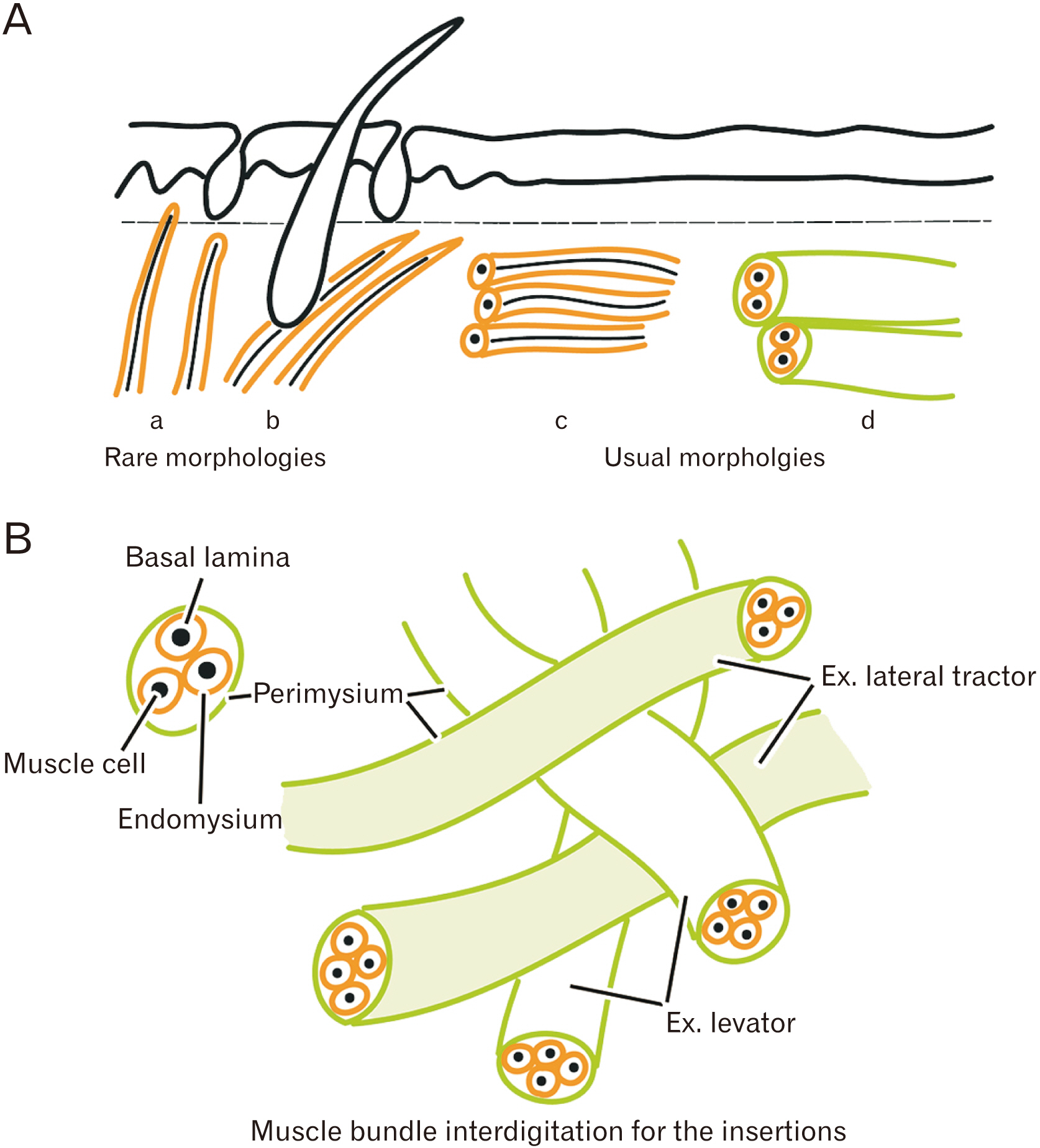
- Striated muscle insertions into the skin and mucosa are present in the head, neck, and pelvic floor. We reexamined the histology of these tissues to elucidate their role in transmission of the force. We examined histological sections of 25 human fetuses (gestational ages of ~11–19 weeks and ~26–40 weeks) and 6 cadavers of elderly individuals. Facial muscle insertion or terminal almost always formed as an interdigitation with another muscle or as a circular arrangement in which muscle fiber insertions were sandwiched and mechanically supported by other muscle fibers (like an in-series muscle). Our examination of the face revealed some limited exceptions in which muscle fibers that approached the dermis were always in the nasalis and mentalis muscles, and often in the levator labii superioris alaeque nasi muscle. The buccinator muscle was consistently inserted into the basement membrane of the oral mucosa. Parts of the uvulae muscle in the soft palate and of the intrinsic vertical muscle of the tongue were likely to direct toward the mucosa. In contrast, the pelvic floor did not contain striated muscle fibers that were directed toward the skin or mucosa. Although ‘cutaneous muscle’ is a common term, the actual insertion of a muscle into the skin or mucosa seemed to be very rare. Instead, superficial muscle insertion often consisted of interdigitated muscle bundles that had different functional vectors. In this case, the terminal of one muscle bundle was sandwiched and fixed mechanically by other bundles.
Figure
Reference
-
References
1. Ohtsuka K, Tomita H, Murakami G. 2002; Anatomy of the tonsillar bed: topographical relationship between the palatine tonsil and the lingual branch of the glossopharyngeal nerve. Acta Otolaryngol Suppl. 546:99–109. DOI: 10.1080/00016480260046472. PMID: 12132628.
Article2. Meng H, Murakami G, Suzuki D, Miyamoto S. 2008; Anatomical variations in stylopharyngeus muscle insertions suggest interindividual and left/right differences in pharyngeal clearance function of elderly patients: a cadaveric study. Dysphagia. 23:251–7. DOI: 10.1007/s00455-007-9131-2. PMID: 18427898.
Article3. Abe S, Fukuda M, Yamane S, Saka H, Katori Y, Rodríguez-Vázquez JF, Murakami G. 2013; Fetal anatomy of the upper pharyngeal muscles with special reference to the nerve supply: is it an enteric plexus or simply an intramuscular nerve? Anat Cell Biol. 46:141–8. DOI: 10.5115/acb.2013.46.2.141. PMID: 23869261. PMCID: PMC3713278.
Article4. Rohan RF, Turner L. 1956; The levator palati muscle. J Anat. 90:153–4. DOI: 10.1097/00006534-196811000-00036. PMID: 13295160. PMCID: PMC1244830.5. Doménech-Ratto G. 1977; Development and peripheral innervation of the palatal muscles. Acta Anat (Basel). 97:4–14. DOI: 10.1159/000144712. PMID: 66841.
Article6. Stål PS, Lindman R. 2000; Characterisation of human soft palate muscles with respect to fibre types, myosins and capillary supply. J Anat. 197(Pt 2):275–90. DOI: 10.1017/S0021878299006627. PMID: 11005719. PMCID: PMC1468126.
Article7. Langdon HL, Klueber K. 1978; The longitudinal fibromuscular component of the soft palate in the fifteen-week human fetus: musculus uvulae and palatine raphe. Cleft Palate J. 15:337–48. PMID: 281277.8. Klueber K, Langdon HL. 1979; Anatomy of musculus levator veli palatini in the 15-week human fetus. Acta Anat (Basel). 105:94–105. DOI: 10.1159/000145113. PMID: 525252.
Article9. Shimokawa T, Yi SQ, Izumi A, Ru F, Akita K, Sato T, Tanaka S. 2004; An anatomical study of the levator veli palatini and superior constrictor with special reference to their nerve supply. Surg Radiol Anat. 26:100–5. DOI: 10.1007/s00276-003-0183-1. PMID: 14586563.
Article10. Hinata N, Murakami G. 2014; The urethral rhabdosphincter, levator ani muscle, and perineal membrane: a review. Biomed Res Int. 2014:906921. DOI: 10.1155/2014/906921. PMID: 24877147. PMCID: PMC4022307.
Article11. Sasaki H, Hinata N, Kurokawa T, Murakami G. 2014; Supportive tissues of the vagina with special reference to a fibrous skeleton in the perineum: a review. Open J Obstet Gynecol. 4:144–57. DOI: 10.4236/ojog.2014.43025.
Article12. Kim JH, Kinugasa Y, Yu HC, Murakami G, Abe S, Cho BH. 2015; Lack of striated muscle fibers in the longitudinal anal muscle of elderly Japanese: a histological study using cadaveric specimens. Int J Colorectal Dis. 30:43–9. DOI: 10.1007/s00384-014-2038-0. PMID: 25331031.
Article13. Arakawa T, Hayashi S, Kinugasa Y, Murakami G, Fujimiya M. 2010; Development of the external anal sphincter with special reference to intergender difference: observations of mid-term fetuses (15-30 weeks of gestation). Okajimas Folia Anat Jpn. 87:49–58. DOI: 10.2535/ofaj.87.49. PMID: 20882767.
Article14. Arakawa T, Hwang SE, Kim JH, Wilting J, Rodríguez-Vázquez JF, Murakami G, Hwang HP, Cho BH. 2016; Fetal growth of the anal sinus and sphincters, especially in relation to anal anomalies. Int J Colorectal Dis. 31:493–502. DOI: 10.1007/s00384-015-2455-8. PMID: 26615552.
Article15. Yamamoto M, Hirota Y, Watanabe G, Taniguchi S, Murakami G, Rodríguez-Vázquez JF, Abe SI. 2024; Development and growth of median structures in the human tongue: a histological study using human fetuses and adult cadavers. Anat Rec (Hoboken). 307:426–41. DOI: 10.1002/ar.25198. PMID: 36939757.
Article16. Ogawa Y, Hinata N, Murakami G, Bando Y, Kitamura K, Hussein AA, Guru K, Abe SI, Fujisawa M. 2018; Aspects of lymphatic vessel configuration of the human male urinary bladder and adjacent organs: a histological basis for understanding the spread of cancer metastases. Transl Res Anat. 11:10–7. DOI: 10.1016/j.tria.2018.05.001.
Article17. Hinata N, Hussein AA, Bando Y, Terakawa T, Murakami G, Yamamoto M, Abe SI, Guru K, Fujisawa M. 2021; Histologic investigation of the female vesicourethral junction and adjacent tissues for nerve-sparing radical cystectomy. Urology. 149:161–7. DOI: 10.1016/j.urology.2020.12.001. PMID: 33309709.
Article18. Huijing PA, van de Langenberg RW, Meesters JJ, Baan GC. 2007; Extramuscular myofascial force transmission also occurs between synergistic muscles and antagonistic muscles. J Electromyogr Kinesiol. 17:680–9. DOI: 10.1016/j.jelekin.2007.02.005. PMID: 17383898.
Article19. Schumann NP, Bongers K, Scholle HC, Guntinas-Lichius O. 2021; Atlas of voluntary facial muscle activation: visualization of surface electromyographic activities of facial muscles during mimic exercises. PLoS One. 16:e0254932. DOI: 10.1371/journal.pone.0254932. PMID: 34280246. PMCID: PMC8289121.
Article20. Mueller N, Trentzsch V, Grassme R, Guntinas-Lichius O, Volk GF, Anders C. 2022; High-resolution surface electromyographic activities of facial muscles during mimic movements in healthy adults: a prospective observational study. Front Hum Neurosci. 16:1029415. DOI: 10.3389/fnhum.2022.1029415. PMID: 36579128. PMCID: PMC9790991.
Article21. Trotter JA. 1993; Functional morphology of force transmission in skeletal muscle. A brief review. Acta Anat (Basel). 146:205–22. DOI: 10.1159/000147459. PMID: 8317197.22. Huijing PA. 1999; Muscle as a collagen fiber reinforced composite: a review of force transmission in muscle and whole limb. J Biomech. 32:329–45. DOI: 10.1016/S0021-9290(98)00186-9. PMID: 10213024.
Article23. Light N, Champion AE. 1984; Characterization of muscle epimysium, perimysium and endomysium collagens. Biochem J. 219:1017–26. DOI: 10.1042/bj2191017. PMID: 6743238. PMCID: PMC1153576.
Article24. Purslow PP. 2002; The structure and functional significance of variations in the connective tissue within muscle. Comp Biochem Physiol A Mol Integr Physiol. 133:947–66. DOI: 10.1016/S1095-6433(02)00141-1. PMID: 12485685.
Article25. Sleboda DA, Stover KK, Roberts TJ. 2020; Diversity of extracellular matrix morphology in vertebrate skeletal muscle. J Morphol. 281:160–9. DOI: 10.1002/jmor.21088. PMID: 31840868. PMCID: PMC7137673.
Article26. Mitz V, Peyronie M. 1976; The superficial musculo-aponeurotic system (SMAS) in the parotid and cheek area. Plast Reconstr Surg. 58:80–8. DOI: 10.1097/00006534-197607000-00013. PMID: 935283.
Article27. Watanabe K, Han A, Inoue E, Iwanaga J, Tabira Y, Yamashita A, Kikuchi K, Haikata Y, Nooma K, Saga T. 2023; The key structure of the facial soft tissue: the superficial musculoaponeurotic system. Kurume Med J. 68:53–61. DOI: 10.2739/kurumemedj.MS682008. PMID: 37062726.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Striated muscle fiber crossings of the head and neck: a histological study using near-term human fetuses and elderly cadavers
- Macrophage density in pharyngeal and laryngeal muscles greatly exceeds that in other striated muscles: an immunohistochemical study using elderly human cadavers
- Reappraisal of intergender differences in the urethral striated sphincter explains why a completely circular arrangement is difficult in females: a histological study using human fetuses
- Anococcygeal Raphe Revisited: A Histological Study Using Mid-Term Human Fetuses and Elderly Cadavers
- Variations of Insertions of the Abductor Pollicis Longus and the Extensor Pollicis Brevis in Korean