Clin Exp Otorhinolaryngol.
2024 May;17(2):147-159. 10.21053/ceo.2023.00079.
Effect of Air Pollutants on Allergic Inflammation in Structural Cells of the Nasal Mucosa
- Affiliations
-
- 1Upper Airway Chronic Inflammatory Diseases Laboratory, Korea University College of Medicine, Seoul, Korea
- 2Medical Device Usability Test Center, Korea University Guro Hospital, Seoul, Korea
- 3Department of Otorhinolaryngology-Head and Neck Surgery, Korea University College of Medicine, Seoul, Korea
- 4Department of Pediatrics, Korea University College of Medicine, Seoul, Korea
- KMID: 2556005
- DOI: http://doi.org/10.21053/ceo.2023.00079
Abstract
Objectives
. Air pollution is an increasing global concern, and its effect on allergic inflammation has attracted the attention of many researchers. Particulate matter (PM) is a major component of ambient air pollution, and heavy metals are the primary toxic constituents of PM. As previous studies on the impact of air pollutants on allergic inflammation did not adequately mimic real-world atmospheric exposure, we developed an experimental model to investigate the effects of aerosolized air pollutants on nasal epithelial cells and fibroblasts.
Methods
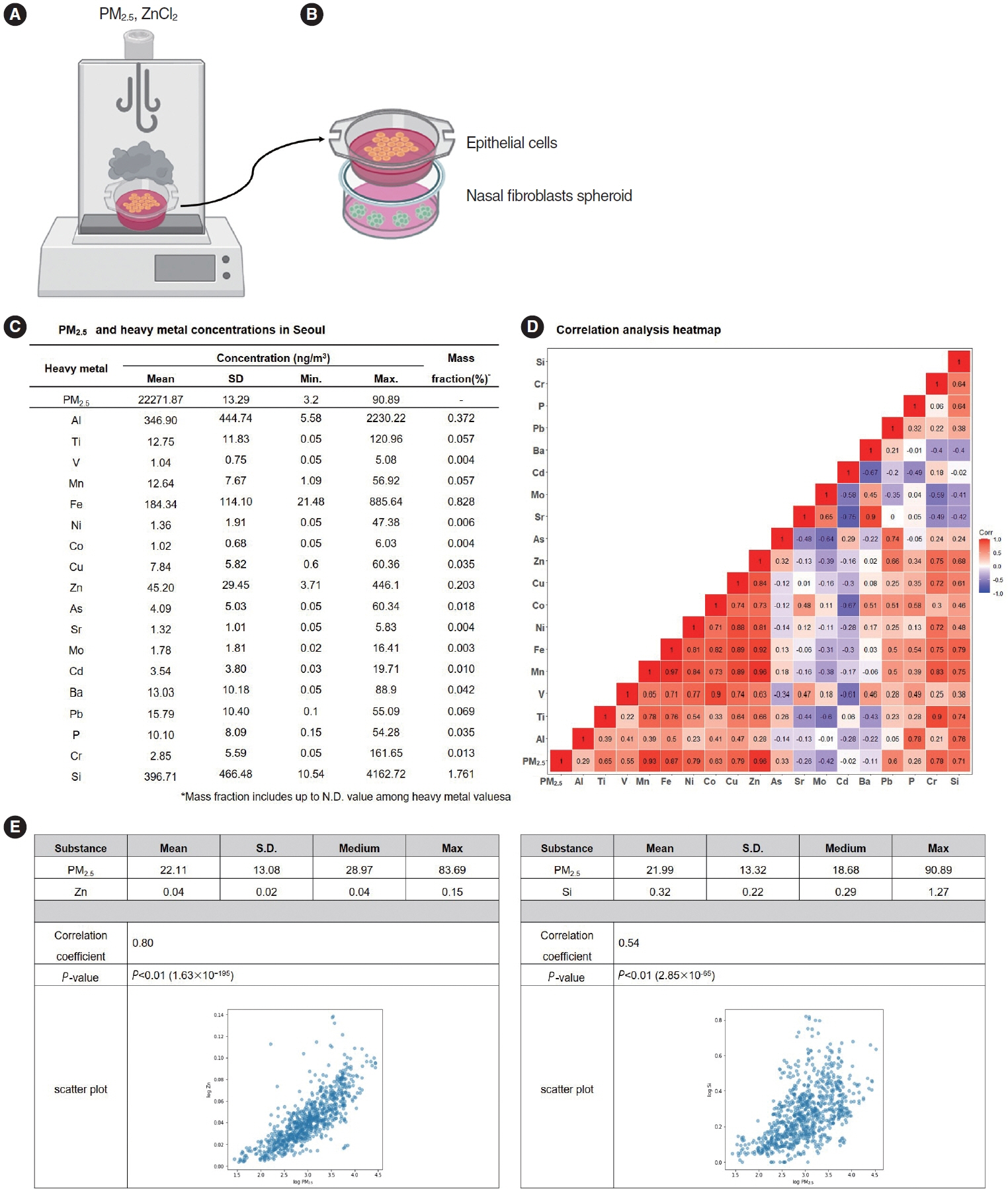
. We collected particulate matter 2.5 (PM2.5) samples from ambient 24-hour air samples obtained in Seoul from August 2020 to August 2022, and then conducted component analysis for metallic constituents. Primary nasal epithelial cells and nasal fibroblasts, obtained and cultured from the turbinate tissues of human participants, were treated with PM2.5. The associations of heavy metals identified from the component analysis with cytokine expression were investigated. A three-dimensional (3D)-hybrid culture model, consisting of co-culture of an air-liquid interface and nasal fibroblast spheroids, was constructed to observe the impact of aerosolized air pollutants.
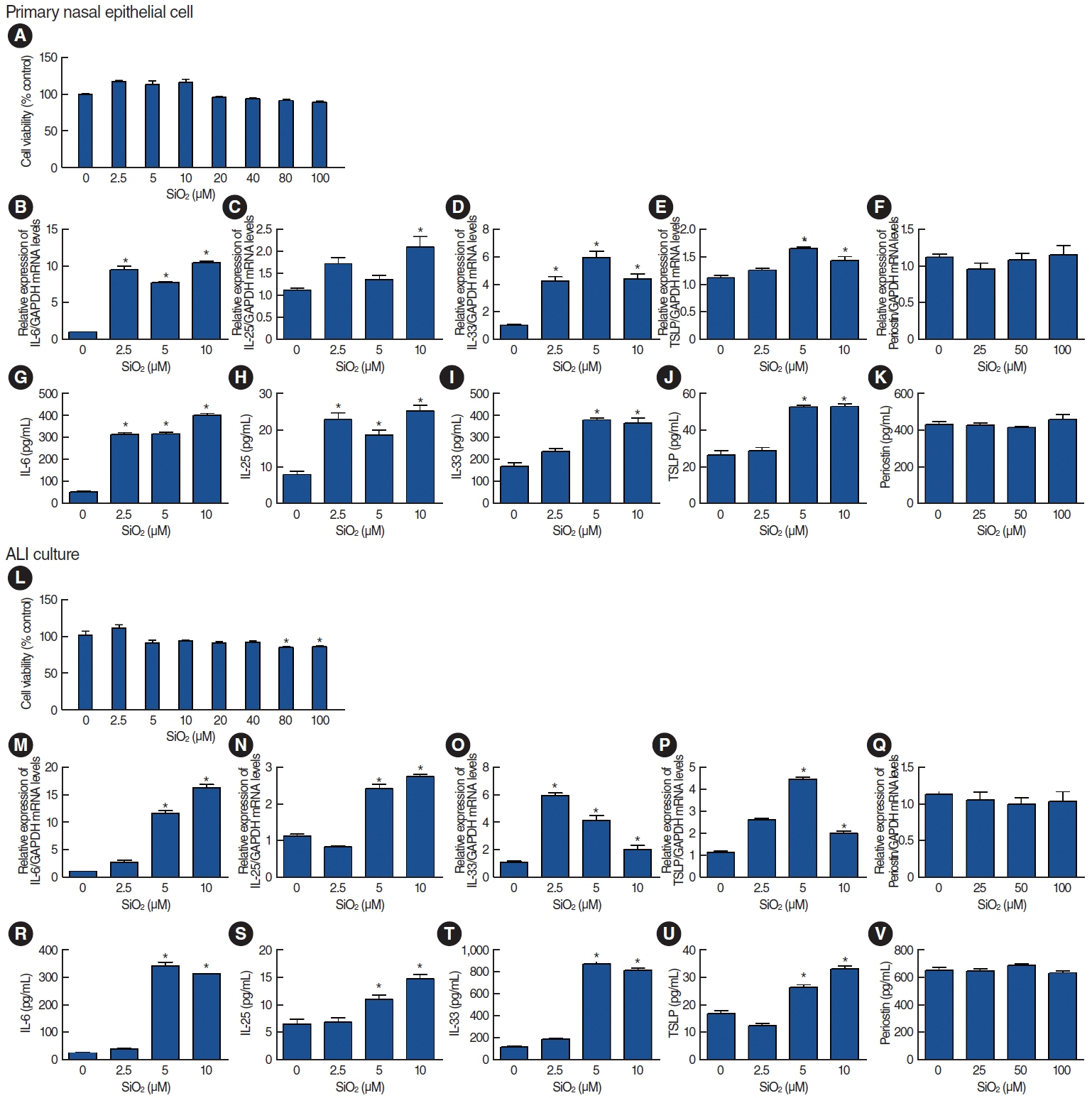
Results
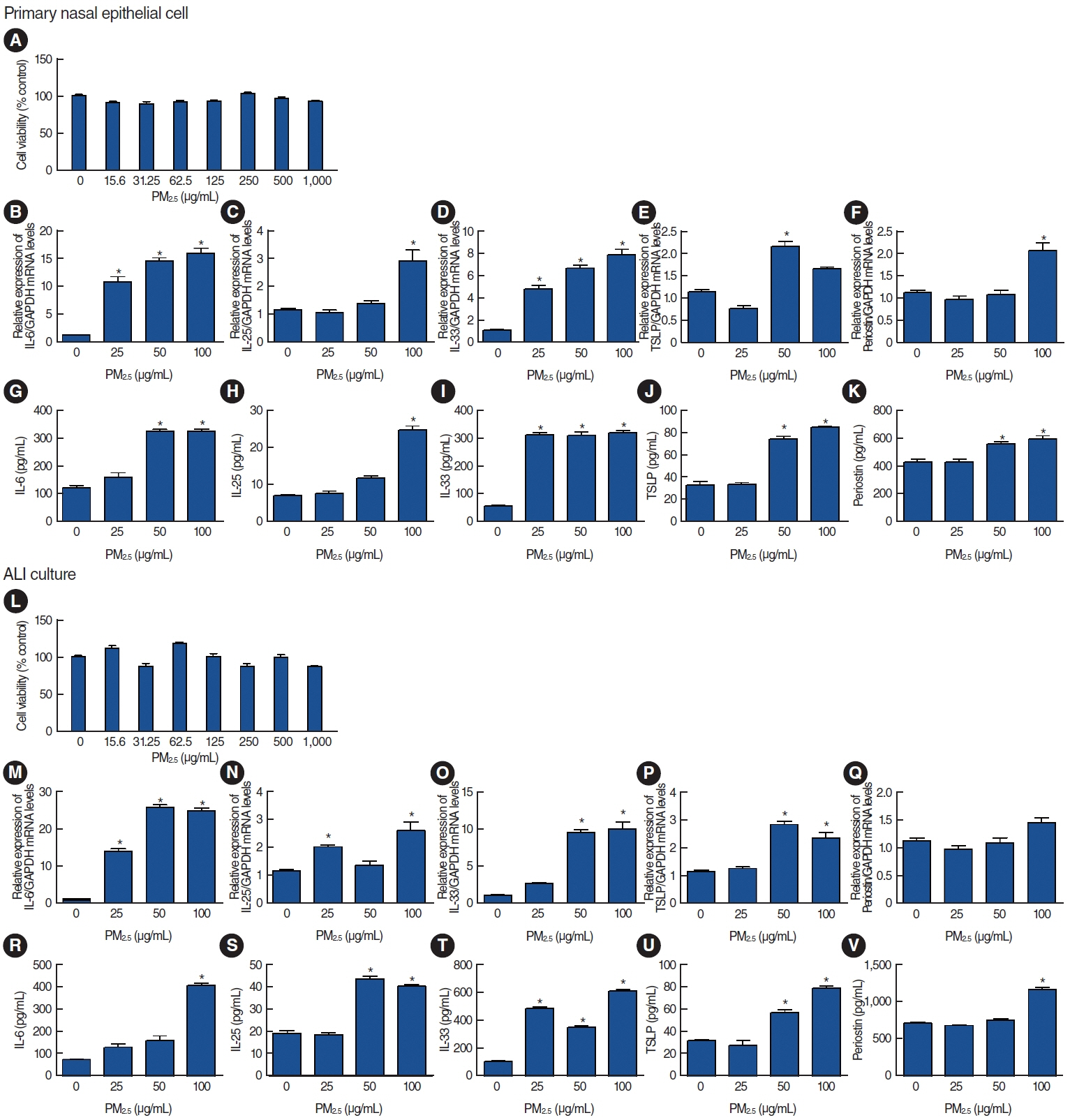
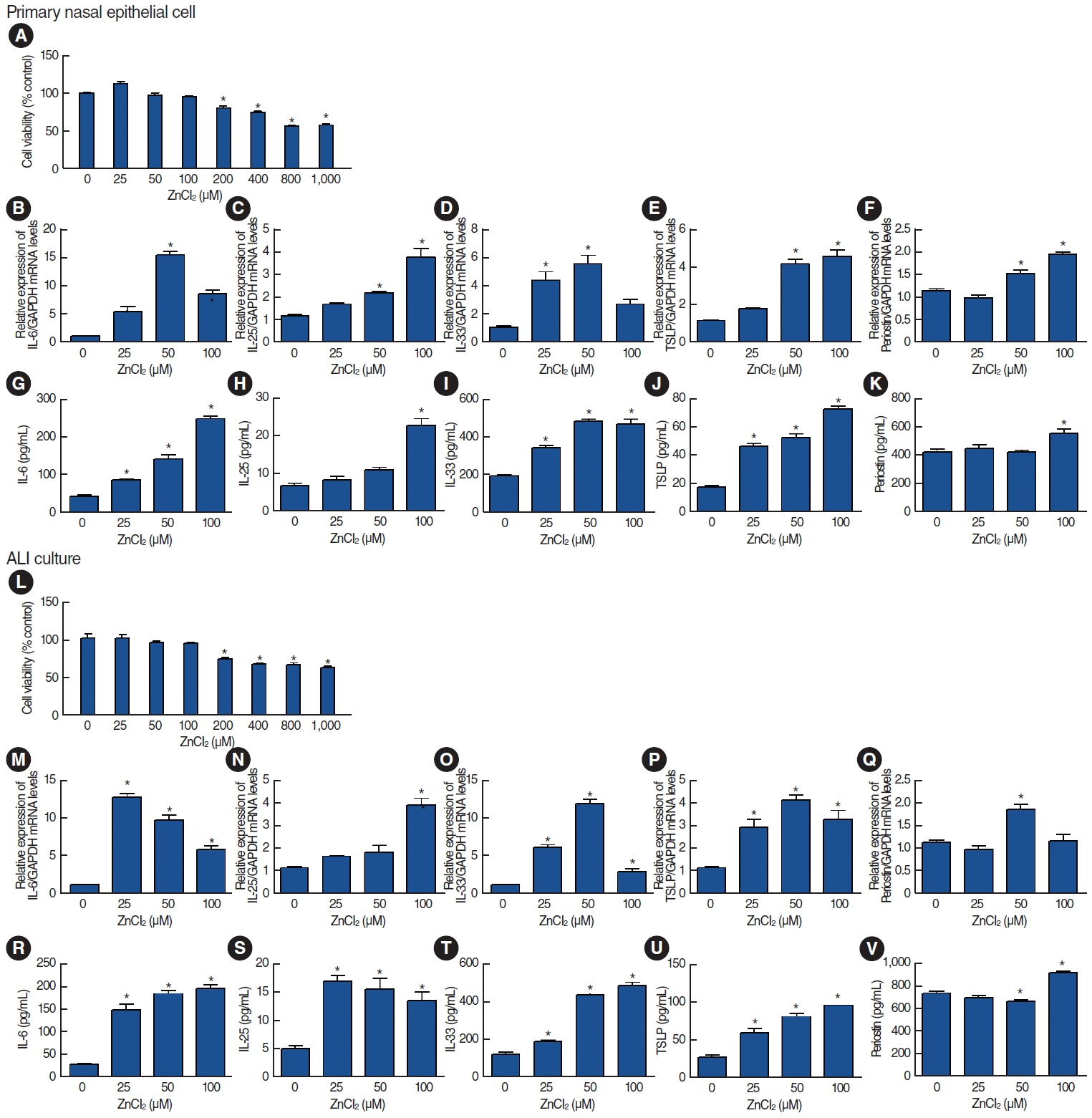
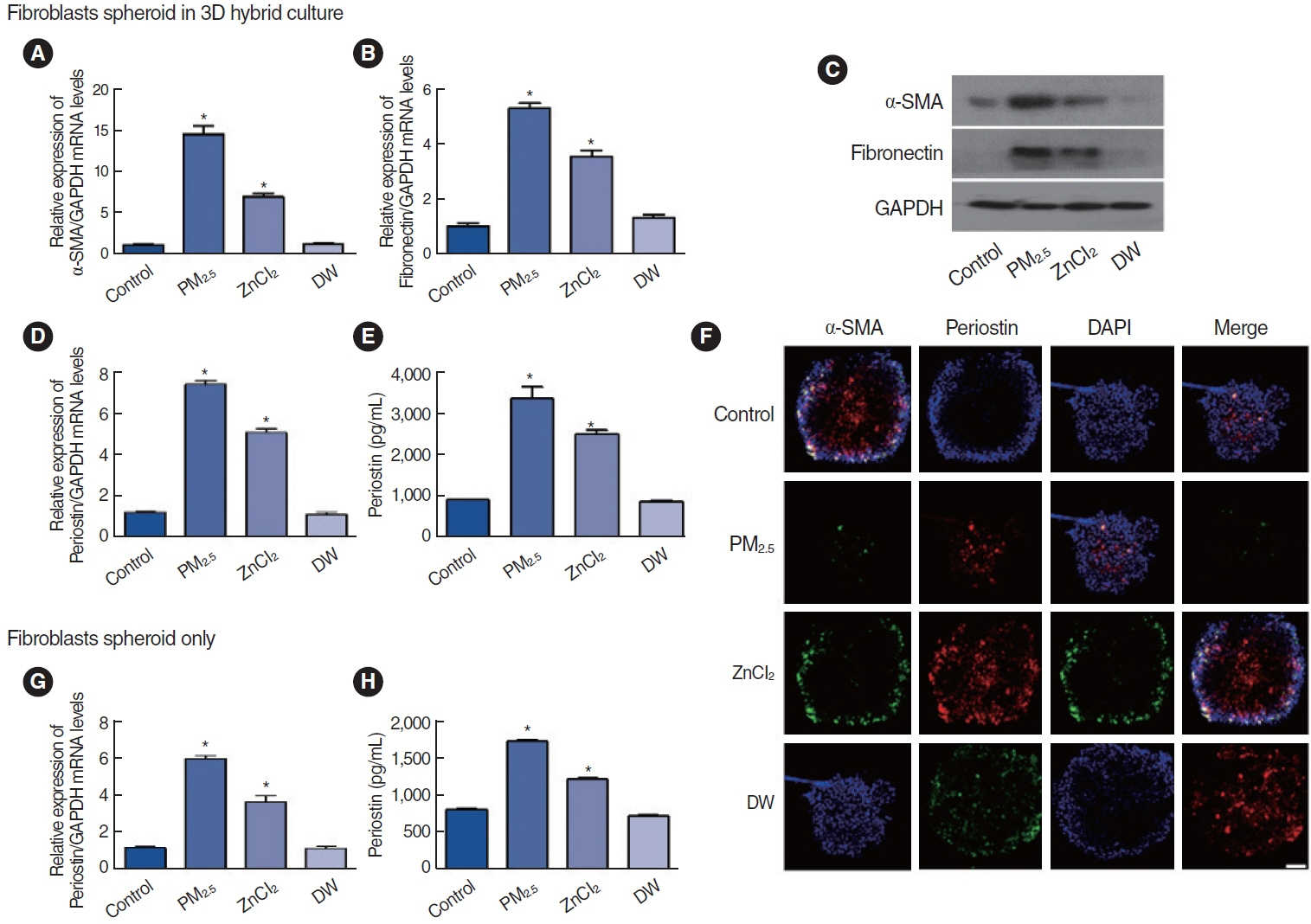
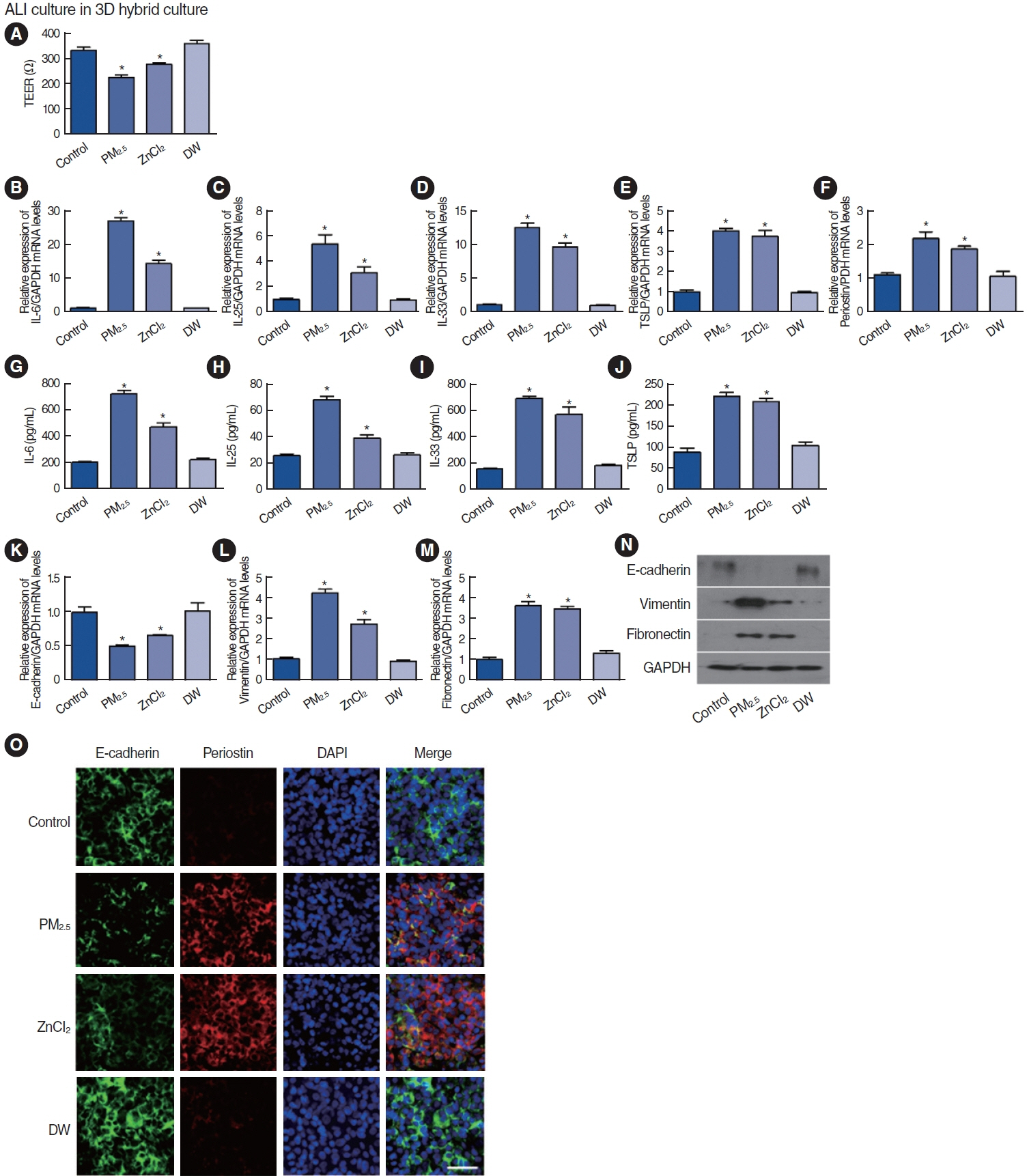
. Among the heavy metals, Si was the predominant component of PM2.5, and Zn showed the highest correlation with the concentration of PM2.5 in Seoul. PM2.5, Zn, and Si increased the production of epithelial cell-derived cytokines, and PM2.5 and Zn exhibited similar trends with one another. Exposure of the 3D-hybrid model to aerosolized PM2.5 and Zn resulted in elevated periostin, alpha-smooth muscle actin, and fibronectin expression in fibroblast spheroids, and those without an epithelial barrier exhibited a similar increase in periostin expression.
Conclusion
. Ambient air pollutants in the form of aerosols increase the expression of allergic inflammatory cytokines in both nasal epithelial cells and fibroblasts. Regulations on air pollution will help reduce the global burden of allergic diseases in the future.
Figure
Reference
-
1. Nguyen TT, Higashi T, Kambayashi Y, Anyenda EO, Michigami Y, Hara J, et al. A longitudinal study of association between heavy metals and itchy eyes, coughing in chronic cough patients: related with nonimmunoglobulin E mediated mechanism. Int J Environ Res Public Health. 2016; Jan. 13(1):110.
Article2. Butt EW, Turnock ST, Rigby R, Reddington CL, Yoshioka M, Johnson JS, et al. Global and regional trends in particulate air pollution and attributable health burden over the past 50 years. Environ Res Lett. 2017; Oct. 12(10):104017.
Article3. Shaddick G, Thomas ML, Mudu P, Ruggeri G, Gumy S. Half the world’s population are exposed to increasing air pollution. NPJ Clim Atmos Sci. 2020; Jun. 3(1):23.
Article4. World Health Organization. WHO global air quality guidelines: particulate matter (PM2.5 and PM10), ozone, nitrogen dioxide, sulfur dioxide and carbon monoxide. World Health Organization;2021.5. Lee BJ, Kim B, Lee K. Air pollution exposure and cardiovascular disease. Toxicol Res. 2014; Jun. 30(2):71–5.
Article6. Lee KI, Chung YJ, Mo JH. The impact of air pollution on allergic rhinitis. Allergy Asthma Respir Dis. 2021; Jan. 9(1):3–11.
Article7. Jeong SH, Kim JH, Son BK, Hong SC, Kim SY, Lee GH, et al. Comparison of air pollution and the prevalence of allergy-related diseases in Incheon and Jeju City. Korean J Pediatr. 2011; Dec. 54(12):501–6.
Article8. Zhang F, Wang W, Lv J, Krafft T, Xu J. Time-series studies on air pollution and daily outpatient visits for allergic rhinitis in Beijing, China. Sci Total Environ. 2011; Jun. 409(13):2486–92.
Article9. Burte E, Leynaert B, Marcon A, Bousquet J, Benmerad M, Bono R, et al. Long-term air pollution exposure is associated with increased severity of rhinitis in 2 European cohorts. J Allergy Clin Immunol. 2020; Mar. 145(3):834–42.
Article10. Aghapour M, Ubags ND, Bruder D, Hiemstra PS, Sidhaye V, Rezaee F, et al. Role of air pollutants in airway epithelial barrier dysfunction in asthma and COPD. Eur Respir Rev. 2022; Mar. 31(163):210112.
Article11. Czarnobilska E, Bulanda M, Bulanda D, Mazur M. The influence of air pollution on the development of allergic inflammation in the airways in Krakow’s atopic and non-atopic residents. J Clin Med. 2021; May. 10(11):2383.
Article12. Lu X, Gong C, Lv K, Zheng L, Li B, Zhao Y, et al. Impacts of combined exposure to formaldehyde and PM2.5 at ambient concentrations on airway inflammation in mice. Environ Pollut. 2022; Dec. 315:120234.
Article13. Li CH, Tsai ML, Chiou HC, Lin YC, Liao WT, Hung CH. Role of macrophages in air pollution exposure related asthma. Int J Mol Sci. 2022; Oct. 23(20):12337.
Article14. Jung HJ, Han DH. Effects of particulate matter on allergic rhinitis and possible mechanisms. Korean J Otorhinolaryngol-Head Neck Surg. 2023; Feb. 66(2):75–84.
Article15. London NR Jr, Ramanathan M Jr. The role of the sinonasal epithelium in allergic rhinitis. Otolaryngol Clin North Am. 2017; Dec. 50(6):1043–50.
Article16. Shin JM, Yang HW, Park JH, Kim TH. Role of nasal fibroblasts in airway remodeling of chronic rhinosinusitis: the modulating functions reexamined. Int J Mol Sci. 2023; Feb. 24(4):4017.
Article17. Izuhara K, Nunomura S, Nanri Y, Ogawa M, Ono J, Mitamura Y, et al. Periostin in inflammation and allergy. Cell Mol Life Sci. 2017; Dec. 74(23):4293–303.
Article18. Sonnenberg-Riethmacher E, Miehe M, Riethmacher D. Periostin in allergy and inflammation. Front Immunol. 2021; Aug. 12:722170.19. Liu AY, Zheng H, Ouyang G. Periostin, a multifunctional matricellular protein in inflammatory and tumor microenvironments. Matrix Biol. 2014; Jul. 37:150–6.
Article20. Takayama G, Arima K, Kanaji T, Toda S, Tanaka H, Shoji S, et al. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol. 2006; Jul. 118(1):98–104.
Article21. Hoshino M, Akitsu K, Kubota K, Ohtawa J. Diagnostic utility of serum periostin for house dust mite sublingual immunotherapy response in allergic rhinitis. European Respiratory Society;2021.22. Krasilnikova SV, Tush EV, Frolov PA, Ovsyannikov DY, Terentyeva AB, Kubysheva NI, et al. Periostin as a biomarker of allergic inflammation in atopic bronchial asthma and allergic rhinitis (a pilot study). Sovrem Tekhnologii Med. 2021; 12(5):37–45.
Article23. Hong Z, Guo Z, Zhang R, Xu J, Dong W, Zhuang G, et al. Airborne fine particulate matter induces oxidative stress and inflammation in human nasal epithelial cells. Tohoku J Exp Med. 2016; Jun. 239(2):117–25.
Article24. Lee DC, Choi H, Oh JM, Hong Y, Jeong SH, Kim CS, et al. The effect of urban particulate matter on cultured human nasal fibroblasts. Int Forum Allergy Rhinol. 2018; Sep. 8(9):993–1000.
Article25. Koh HY, Kim TH, Sheen YH, Lee SW, An J, Kim MA, et al. Serum heavy metal levels are associated with asthma, allergic rhinitis, atopic dermatitis, allergic multimorbidity, and airflow obstruction. J Allergy Clin Immunol Pract. 2019; Nov-Dec. 7(8):2912–5.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Influence of the Sick House Syndrome on Nasal Mucosa and Nasal Symptoms
- Between Air Pollutants and Prevalence of Allergic Disease
- Air pollution and childhood allergic disease
- Expression of MMP-9 and TIMP-1 in the Nasal Mucosa of Allergic Rhinitis
- Expression of HSP72 in Nasal Mucosa of Patients with Allergic Rhinitis