Clin Transplant Res.
2024 Mar;38(1):1-6. 10.4285/ctr.23.0069.
New treatment for antibody-mediated rejection: interleukin-6 inhibitors
- Affiliations
-
- 1Division of Nephrology, Department of Internal Medicine, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan, Korea
- 2Transplantation Research Institute, Kosin University College of Medicine, Busan, Korea
- KMID: 2555990
- DOI: http://doi.org/10.4285/ctr.23.0069
Abstract
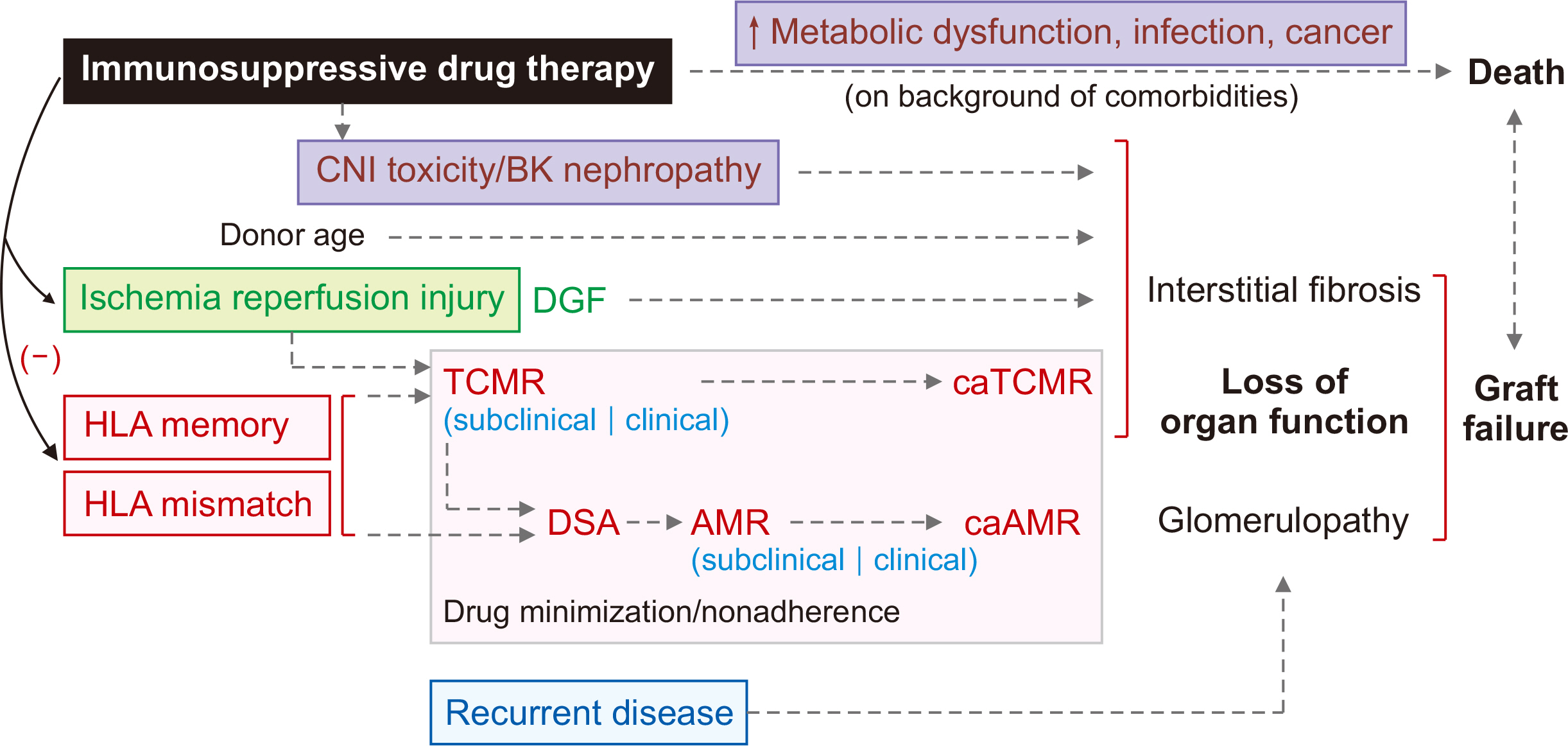
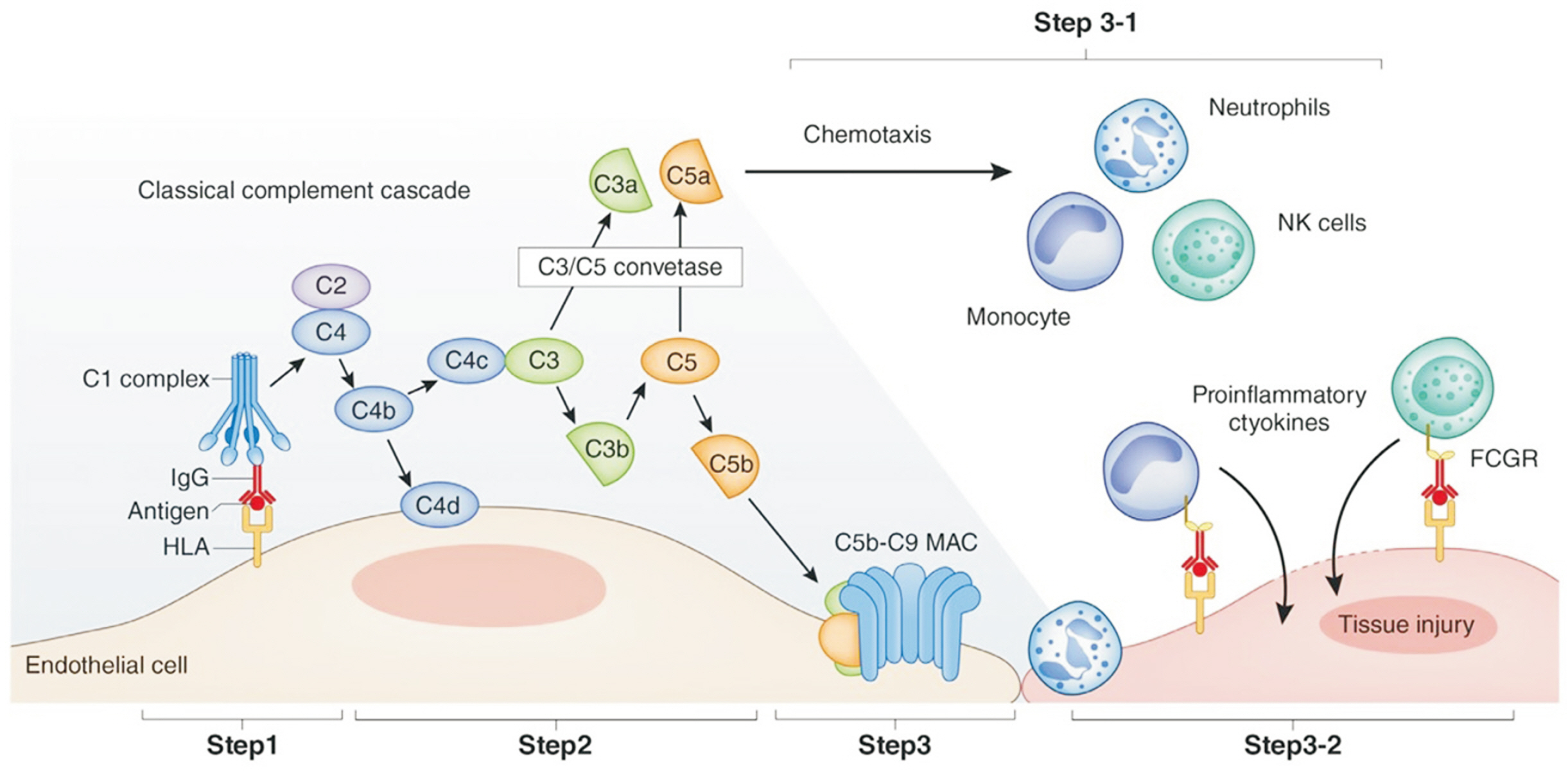
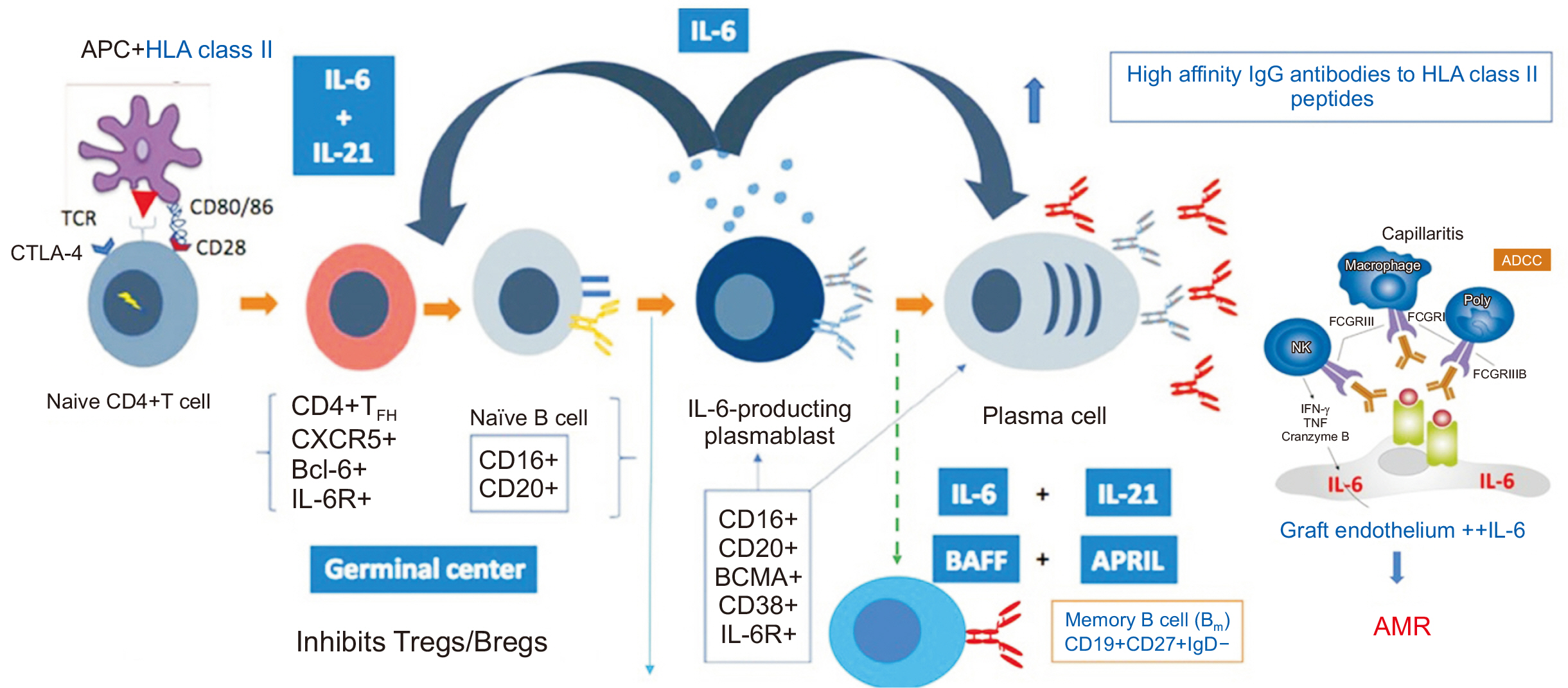
- Following kidney transplantation, antibody-mediated rejection (AMR) occurs when the antibodies of the immune system attack the transplanted organ, leading to damage of the kidney tissue. De novo human leukocyte antigen donor-specific antibodies (HLADSAs) play a key role in AMR. Current therapeutic approaches include intravenous immunoglobulin, anti-CD20 antibodies, and plasmapheresis. In cases resistant to treatment, proteasome inhibitors and C5 inhibitors may be employed. Nevertheless, a pressing need exists for new medications to improve transplant survival and reduce complications. In the context of AMR, interleukin (IL)-6 is instrumental in the development and maturation of B cells into plasma cells, which then produce HLA-DSAs targeting the allograft. IL-6 inhibitors are currently under investigation and show promise due to the essential role of IL-6 in the immune response; however, additional research is necessary.
Keyword
Figure
Reference
-
1. Borski A, Eskandary F, Haindl S, Doberer K, Mühlbacher J, Mayer KA, et al. 2023; Anti-interleukin-6 antibody clazakizumab in antibody-mediated renal allograft rejection: accumulation of antibody-neutralized interleukin-6 without signs of proinflammatory rebound phenomena. Transplantation. 107:495–503. DOI: 10.1097/TP.0000000000004285. PMID: 35969004.
Article2. Sasaki H, Tanabe T, Tsuji T, Hotta K. 2023; Mechanism and treatment for chronic antibody-mediated rejection in kidney transplant recipients. Int J Urol. 30:624–33. DOI: 10.1111/iju.15197. PMID: 37306194.
Article3. Alasfar S, Kodali L, Schinstock CA. 2023; Current therapies in kidney transplant rejection. J Clin Med. 12:4927. DOI: 10.3390/jcm12154927. PMID: 37568328. PMCID: PMC10419508.
Article4. The Korean Society of Nephrology. Clinical nephrology. 3rd ed. The Korean Society of Nephrology;2022.5. Doberer K, Duerr M, Halloran PF, Eskandary F, Budde K, Regele H, et al. 2021; A randomized clinical trial of anti-il-6 antibody clazakizumab in late antibody-mediated kidney transplant rejection. J Am Soc Nephrol. 32:708–22. DOI: 10.1681/ASN.2020071106. PMID: 33443079. PMCID: PMC7920172.
Article6. López Del Moral C, Wu K, Naik M, Osmanodja B, Akifova A, Lachmann N, et al. 2022; The natural history of de novo donor-specific HLA antibodies after kidney transplantation. Front Med (Lausanne). 9:943502. DOI: 10.3389/fmed.2022.943502. PMID: 36186822. PMCID: PMC9523126.
Article7. Kim MY, Brennan DC. 2021; Therapies for chronic allograft rejection. Front Pharmacol. 12:651222. DOI: 10.3389/fphar.2021.651222. PMID: 33935762. PMCID: PMC8082459.
Article8. Sharma R. 2022; Anti-interleukin 6 therapeutics for chronic antibody-mediated rejection in kidney transplant recipients. Exp Clin Transplant. 20:709–16. DOI: 10.6002/ect.2021.0254. PMID: 34981708.
Article9. Hara S. 2023; The chronology of renal allograft dysfunction: the pathological perspectives. Nephron. 147 Suppl 1:67–73. DOI: 10.1159/000531575. PMID: 37573772.
Article10. Chancay J, Liu C, Chauhan K, Andersen L, Harris C, Coca S, et al. 2022; Role of time from transplantation to biopsy in histologic ABMR: a single center report. Clin Transplant. 36:e14802. DOI: 10.1111/ctr.14802. PMID: 36069577. PMCID: PMC10211409.
Article11. Tufan Pekkucuksen N, Sigler KE, Akcan Arikan A, Srivaths P. 2021; Tandem plasmapheresis and continuous kidney replacement treatment in pediatric patients. Pediatr Nephrol. 36:1273–8. DOI: 10.1007/s00467-020-04769-z. PMID: 33108508. PMCID: PMC7588944.
Article12. Halloran PF, Madill-Thomsen KS, Pon S, Sikosana ML, Böhmig GA, Bromberg J, et al. 2022; Molecular diagnosis of ABMR with or without donor-specific antibody in kidney transplant biopsies: differences in timing and intensity but similar mechanisms and outcomes. Am J Transplant. 22:1976–91. DOI: 10.1111/ajt.17092. PMID: 35575435. PMCID: PMC9540308.
Article13. Jordan SC, Ammerman N, Huang E, Vo A. 2022; Importance of IL-6 inhibition in prevention and treatment of antibody-mediated rejection in kidney allografts. Am J Transplant. 22 Suppl 4:28–37. DOI: 10.1111/ajt.17207. PMID: 36453709.
Article14. Miller CL, Madsen JC. 2021; IL-6 directed therapy in transplantation. Curr Transplant Rep. 8:191–204. DOI: 10.1007/s40472-021-00331-4. PMID: 34099967. PMCID: PMC8173333.
Article15. Boonpheng B, Hansrivijit P, Thongprayoon C, Mao SA, Vaitla PK, Bathini T, et al. 2021; Rituximab or plasmapheresis for prevention of recurrent focal segmental glomerulosclerosis after kidney transplantation: a systematic review and meta-analysis. World J Transplant. 11:303–19. DOI: 10.5500/wjt.v11.i7.303. PMID: 34316454. PMCID: PMC8291000.
Article16. Ulisses LR, Paixão JO, Agena F, Souza PS, Paula FJ, Bezerra G, et al. 2022; Desensitization using IVIG alone for living-donor kidney transplant: impact on donor-specific antibodies. J Bras Nefrol. 44:527–32. DOI: 10.1590/2175-8239-jbn-2021-0200. PMID: 35438714. PMCID: PMC9838666.
Article17. Boonpheng B, De Castro IC, Ng YH, Blosser C, Bakthavatsalam R, Gimferrer I, et al. 2023; Tocilizumab for treatment of chronic active antibody-mediated rejection in kidney transplant recipients. Clin Transplant. 37:e14936. DOI: 10.1111/ctr.14936. PMID: 36787372.
Article18. Rostaing LP, Böhmig GA, Gibbons B, Taqi MM. 2023; Post-transplant surveillance and management of chronic active antibody-mediated rejection in renal transplant patients in Europe. Transpl Int. 36:11381. DOI: 10.3389/ti.2023.11381. PMID: 37529383. PMCID: PMC10389272.
Article19. Abuazzam F, Dubrawka C, Abdulhadi T, Amurao G, Alrata L, Yaseen Alsabbagh D, et al. 2023; Emerging therapies for antibody-mediated rejection in kidney transplantation. J Clin Med. 12:4916. DOI: 10.3390/jcm12154916. PMID: 37568318. PMCID: PMC10419906.
Article20. Berger M, Baliker M, Van Gelder T, Böhmig GA, Mannon RB, Kumar D, et al. Chronic active antibody-mediated rejection: opportunity to determine the role of interleukin-6 blockade. Transplantation. 2023; Nov. 9. [Epub]. https://doi.org/10.1097/TP.0000000000004822. DOI: 10.1097/TP.0000000000004822. PMID: 37941113.
Article21. Degner KR, Wilson NA, Reese SR, Parajuli S, Aziz F, Garg N, et al. 2020; Short-term immunopathological changes associated with pulse steroids/IVIG/rituximab therapy in late kidney allograft antibody mediated rejection. Kidney360. 1:389–98. DOI: 10.34067/KID.0001082019. PMID: 34476406. PMCID: PMC8409258.
Article22. Nickerson PW, Böhmig GA, Chadban S, Kumar D, Mannon RB, van Gelder T, et al. 2022; Clazakizumab for the treatment of chronic active antibody-mediated rejection (AMR) in kidney transplant recipients: phase 3 IMAGINE study rationale and design. Trials. 23:1042. DOI: 10.1186/s13063-022-06897-3. PMID: 36550562. PMCID: PMC9772593.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of High Dose Intravenous Immunoglobulins on the Treatment of Antibody Mediated Humoral Rejection and BK Virus Infection in Renal Transplant Recipients
- The Diagnosis of Acute Antibody-Mediated Rejection in ABO-Incompatible Liver Transplants
- Treatment of Refractory Antibody-mediated Rejection with Bortezomib in a Kidney Transplant Recipient: A Case Report
- Pathologic Updates on Antibody Mediated Rejection in Renal Transplantation
- Contribution of long-lived plasma cells to antibody-mediated allograft rejection