Ann Hepatobiliary Pancreat Surg.
2024 May;28(2):238-247. 10.14701/ahbps.23-129.
Safety and efficacy of early corticosteroid withdrawal in liver transplant recipients: A randomized controlled trial
- Affiliations
-
- 1Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Department of Surgery, Seoul National University College of Medicine, Seoul, Korea
- 3Department of Surgery, Catholic University of Daegu College of Medicine, Daegu, Korea
- 4Department of Liver Transplantation and Hepatobiliary Surgery, Ajou University School of Medicine, Suwon, Korea
- KMID: 2555934
- DOI: http://doi.org/10.14701/ahbps.23-129
Abstract
- Backgrounds/Aims
Prolonged use of steroids after liver transplantation (LT) significantly increases the risk of diabetes or cardiovascular disease, which can adversely affect patient outcomes. Our study evaluated the effectiveness and safety of early steroid withdrawal within the first year following LT.
Methods
This study was conducted as an open-label, multicenter, randomized controlled trial. Liver transplant recipients were randomly assigned to one of the following two groups: Group 1, in which steroids were withdrawn two weeks posttransplantation, and Group 2, in which steroids were withdrawn three months posttransplantation. This study included participants aged 20 to 70 years who were scheduled to undergo a single-organ liver transplant from a living or deceased donor at one of the four participating centers.
Results
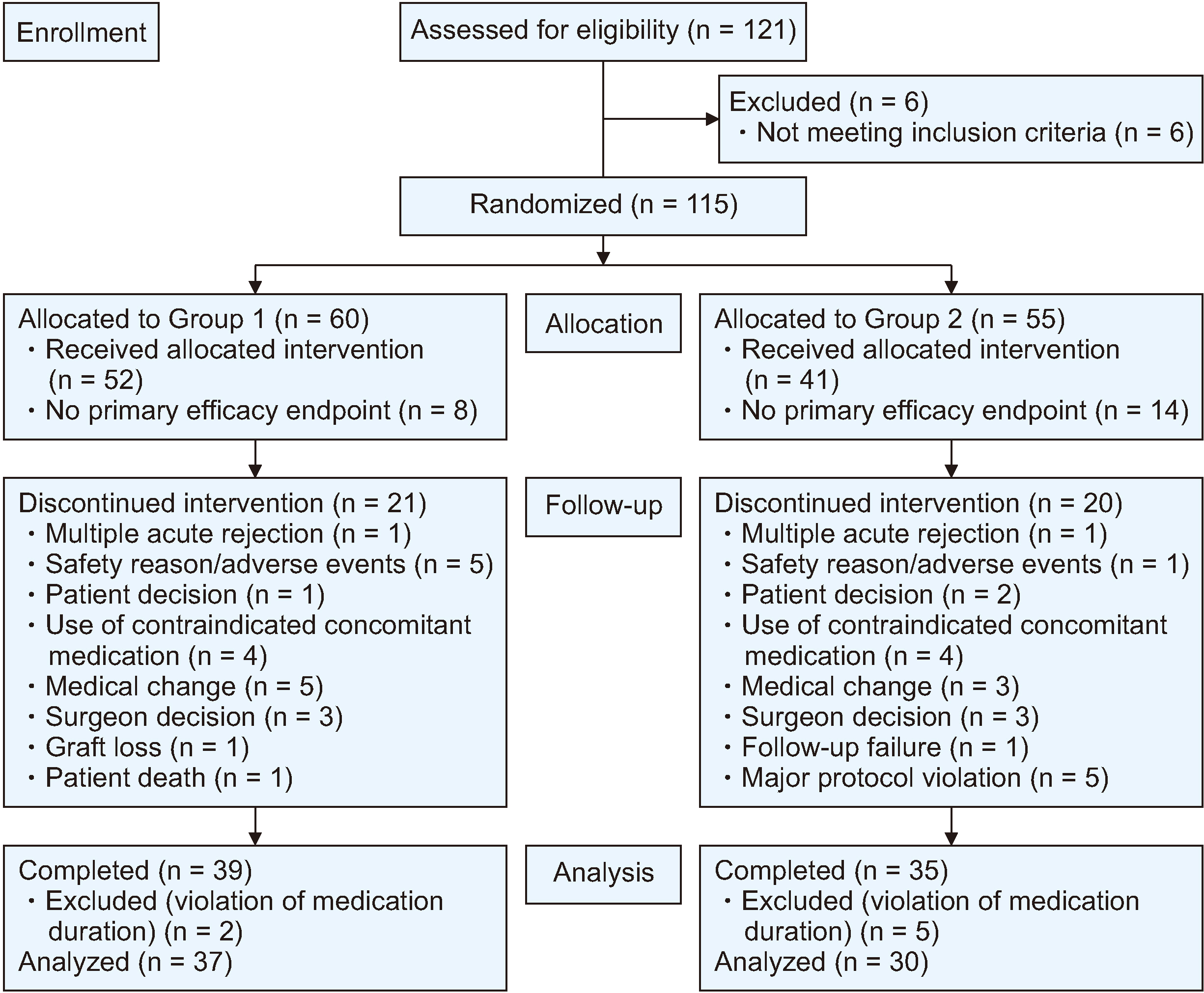
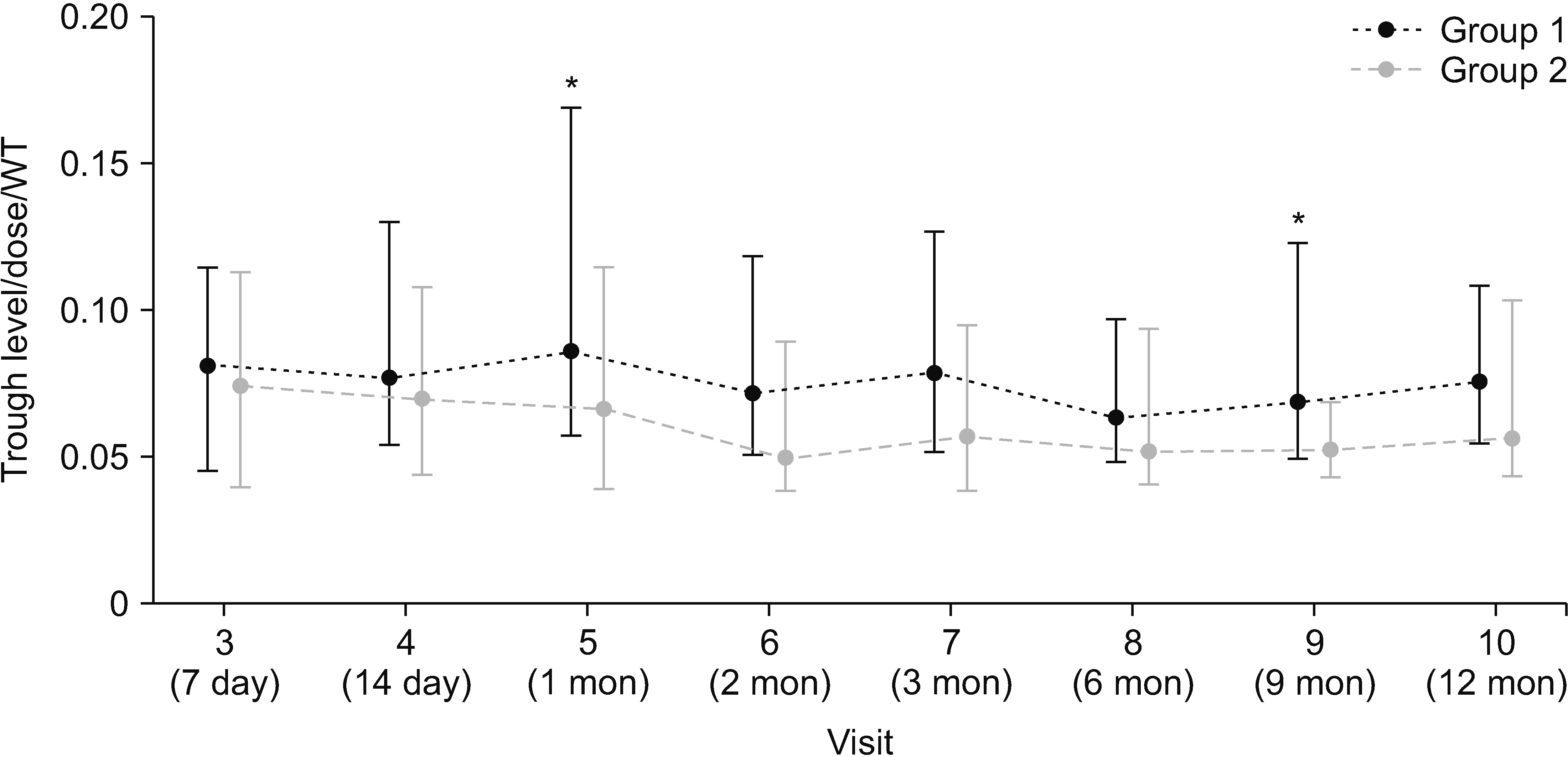
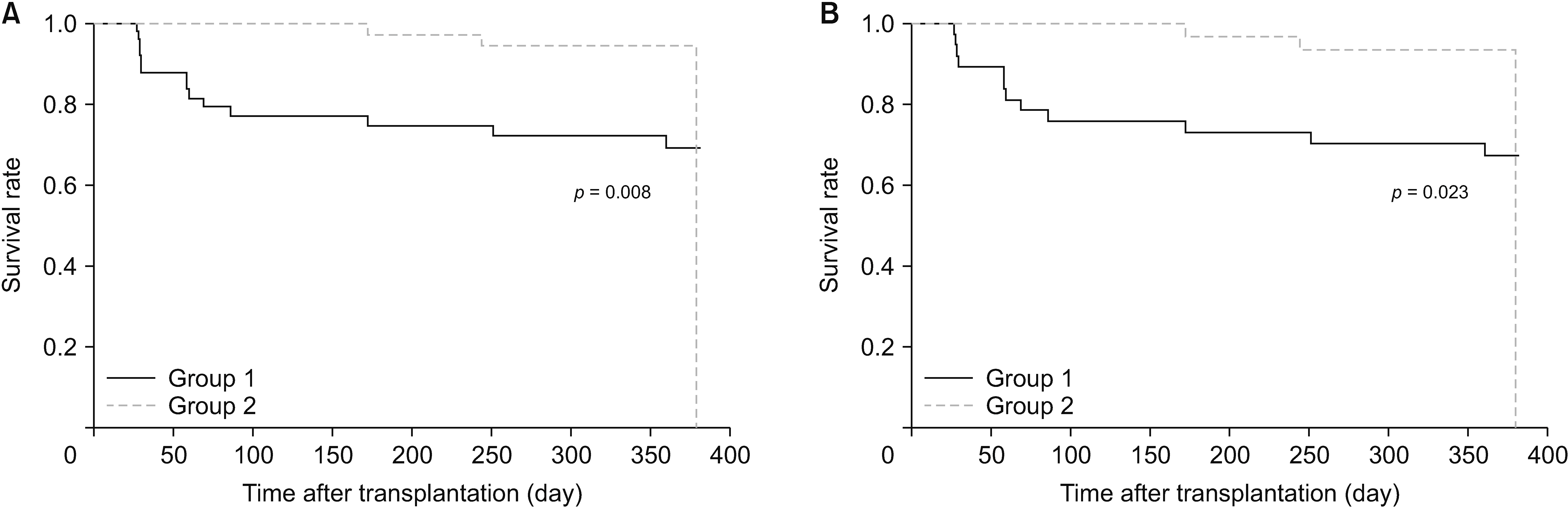
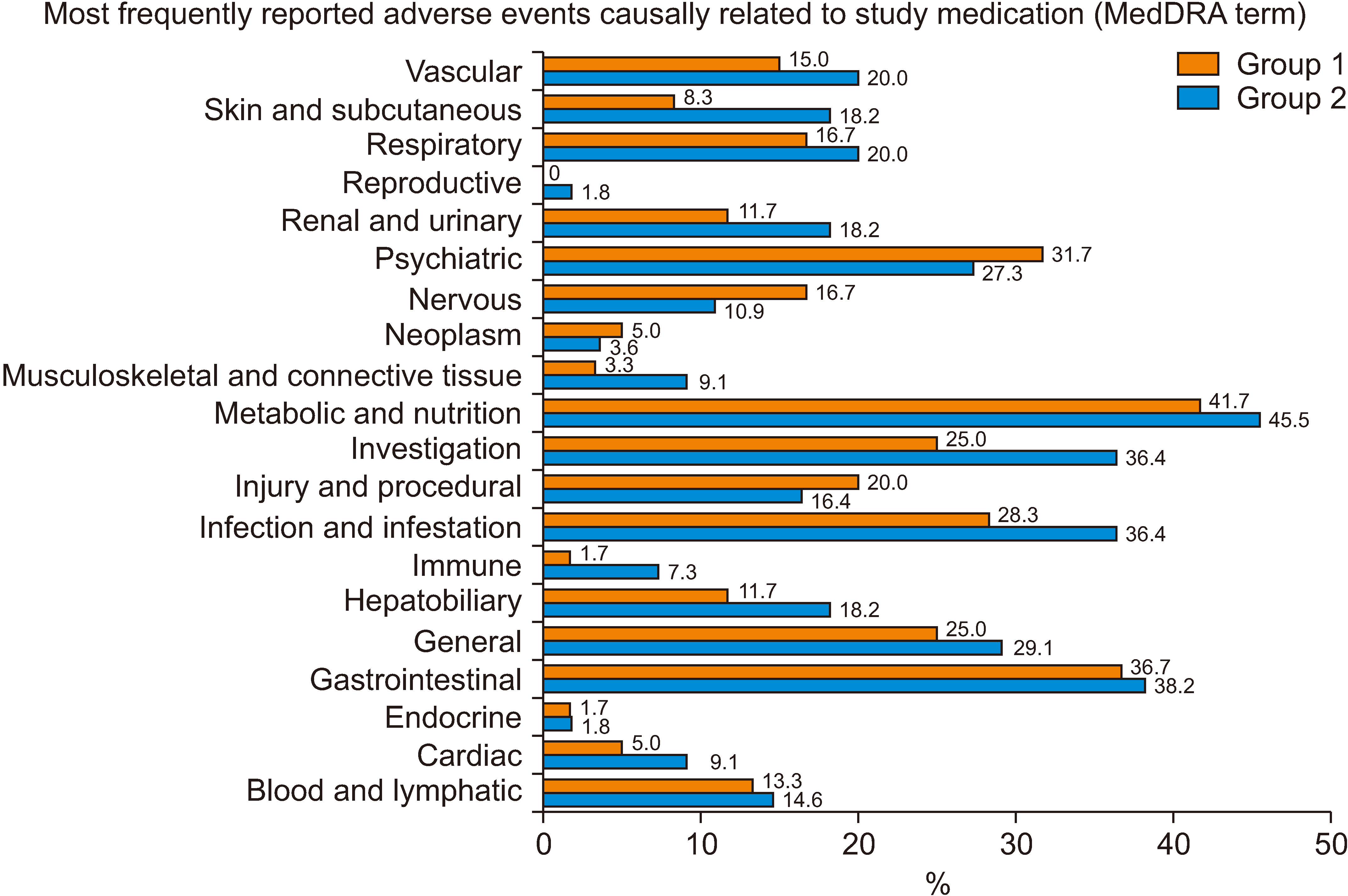
Between November 2012 and August 2020, 115 patients were selected and randomized into two groups, with 60 in Group 1 and 55 in Group 2. The incidence of new-onset diabetes after transplantation (NODAT) was notably higher in Group 1 (32.4%) than in Group 2 (10.0%) in the per-protocol set. Although biopsy-proven acute rejection, graft failure, and mortality did not occur, the median tacrolimus trough level/dose/weight in Group 1 exceeded that in Group 2. No significant differences in safety parameters, such as infection and recurrence of hepatocellular carcinoma, were observed between the two groups.
Conclusions
The present study did not find a significant reduction in the incidence of NODAT in the early steroid withdrawal group. Our study suggests that steroid withdrawal three months posttransplantation is a standard and safe immunosuppressive strategy for LT patients.
Figure
Reference
-
References
1. Fairfield CJ, Harrison EM, Wigmore SJ, VanWagner LB. 2020; Steroid-free versus steroid-containing immunosuppression for liver transplant recipients. Clin Liver Dis (Hoboken). 16:191–195. DOI: 10.1002/cld.992. PMID: 33318786. PMCID: PMC7727845.
Article2. Gu J, Wu X, Lu L, Zhang S, Bai J, Wang J, et al. 2014; Role of steroid minimization in the tacrolimus-based immunosuppressive regimen for liver transplant recipients: a systematic review and meta-analysis of prospective randomized controlled trials. Hepatol Int. 8:198–215. DOI: 10.1007/s12072-014-9523-y. PMID: 24765218. PMCID: PMC3990862.
Article3. Fairfield C, Penninga L, Powell J, Harrison EM, Wigmore SJ. 2018; Glucocorticosteroid-free versus glucocorticosteroid-containing immunosuppression for liver transplanted patients. Cochrane Database Syst Rev. 4:CD007606. DOI: 10.1002/14651858.CD007606.pub4. PMID: 29630730. PMCID: PMC6494590.
Article4. Li DW, Lu TF, Hua XW, Dai HJ, Cui XL, Zhang JJ, et al. 2015; Risk factors for new onset diabetes mellitus after liver transplantation: a meta-analysis. World J Gastroenterol. 21:6329–6340. DOI: 10.3748/wjg.v21.i20.6329. PMID: 26034369. PMCID: PMC4445111.
Article5. Wallia A, Illuri V, Molitch ME. 2016; Diabetes care after transplant: definitions, risk factors, and clinical management. Med Clin North Am. 100:535–550. DOI: 10.1016/j.mcna.2016.01.005. PMID: 27095644.6. Ahmed SH, Biddle K, Augustine T, Azmi S. 2020; Post-transplantation diabetes mellitus. Diabetes Ther. 11:779–801. DOI: 10.1007/s13300-020-00790-5. PMID: 32095994. PMCID: PMC7136383.
Article7. Moon JI, Barbeito R, Faradji RN, Gaynor JJ, Tzakis AG. 2006; Negative impact of new-onset diabetes mellitus on patient and graft survival after liver transplantation: long-term follow up. Transplantation. 82:1625–1628. DOI: 10.1097/01.tp.0000250361.60415.96. PMID: 17198248.
Article8. Baid S, Cosimi AB, Farrell ML, Schoenfeld DA, Feng S, Chung RT, et al. 2001; Posttransplant diabetes mellitus in liver transplant recipients: risk factors, temporal relationship with hepatitis C virus allograft hepatitis, and impact on mortality. Transplantation. 72:1066–1072. DOI: 10.1097/00007890-200109270-00015. PMID: 11579302.
Article9. Lv C, Zhang Y, Chen X, Huang X, Xue M, Sun Q, et al. 2015; New-onset diabetes after liver transplantation and its impact on complications and patient survival. J Diabetes. 7:881–890. DOI: 10.1111/1753-0407.12275. PMID: 25676209.
Article10. Sarno G, Mehta RJ, Guardado-Mendoza R, Jimenez-Ceja LM, De Rosa P, Muscogiuri G. 2013; New-onset diabetes mellitus: predictive factors and impact on the outcome of patients undergoing liver transplantation. Curr Diabetes Rev. 9:78–85. DOI: 10.2174/157339913804143234. PMID: 22974360.
Article11. Yagi S, Kaido T, Iida T, Yoshizawa A, Okajima H, Uemoto S. 2017; New-onset diabetes mellitus after living-donor liver transplantation: association with graft synthetic function. Surg Today. 47:733–742. DOI: 10.1007/s00595-016-1444-z. PMID: 27837276.
Article12. Gebhardt S, Jara M, Malinowski M, Seehofer D, Puhl G, Pratschke J, et al. 2015; Risk factors of metabolic disorders after liver transplantation: an analysis of data from fasted patients. Transplantation. 99:1243–1249. DOI: 10.1097/TP.0000000000000499. PMID: 25539465.13. Parekh J, Corley DA, Feng S. 2012; Diabetes, hypertension and hyperlipidemia: prevalence over time and impact on long-term survival after liver transplantation. Am J Transplant. 12:2181–2187. DOI: 10.1111/j.1600-6143.2012.04077.x. PMID: 22548965.
Article14. Ling Q, Xu X, Xie H, Wang K, Xiang P, Zhuang R, et al. 2016; New-onset diabetes after liver transplantation: a national report from China Liver Transplant Registry. Liver Int. 36:705–712. DOI: 10.1111/liv.13042. PMID: 26681557.
Article15. Kuo HT, Sampaio MS, Ye X, Reddy P, Martin P, Bunnapradist S. 2010; Risk factors for new-onset diabetes mellitus in adult liver transplant recipients, an analysis of the Organ Procurement and Transplant Network/United Network for Organ Sharing database. Transplantation. 89:1134–1140. DOI: 10.1097/TP.0b013e3181d2fec1. PMID: 20386364.
Article16. Xu X, Ling Q, He ZL, Gao F, Zheng SS. 2008; Post-transplant diabetes mellitus in liver transplantation: Hangzhou experience. Hepatobiliary Pancreat Dis Int. 7:465–470.17. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. 1999; A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 130:461–470. DOI: 10.7326/0003-4819-130-6-199903160-00002. PMID: 10075613.
Article18. Hartog H, May CJ, Corbett C, Phillips A, Tomlinson JW, Mergental H, et al. 2015; Early occurrence of new-onset diabetes after transplantation is related to type of liver graft and warm ischaemic injury. Liver Int. 35:1739–1747. DOI: 10.1111/liv.12706. PMID: 25349066.
Article19. Song JL, Gao W, Zhong Y, Yan LN, Yang JY, Wen TF, et al. 2016; Minimizing tacrolimus decreases the risk of new-onset diabetes mellitus after liver transplantation. World J Gastroenterol. 22:2133–2141. DOI: 10.3748/wjg.v22.i6.2133. PMID: 26877618. PMCID: PMC4726686.
Article20. Kim YK, Lee KW, Kim SH, Cho SY, Han SS, Park SJ. 2012; Early steroid withdrawal regimen prevents new-onset diabetes mellitus in old-age recipients after living donor liver transplantation. World J Surg. 36:2443–2448. DOI: 10.1007/s00268-012-1661-6. PMID: 22674089.
Article21. Sgourakis G, Radtke A, Fouzas I, Mylona S, Goumas K, Gockel I, et al. 2009; Corticosteroid-free immunosuppression in liver transplantation: a meta-analysis and meta-regression of outcomes. Transpl Int. 22:892–905. DOI: 10.1111/j.1432-2277.2009.00893.x. PMID: 19453997.
Article22. Segev DL, Sozio SM, Shin EJ, Nazarian SM, Nathan H, Thuluvath PJ, et al. 2008; Steroid avoidance in liver transplantation: meta-analysis and meta-regression of randomized trials. Liver Transpl. 14:512–525. DOI: 10.1002/lt.21396. PMID: 18383081.
Article23. Zhang Z, Sun J, Guo M, Yuan X. 2023; Progress of new-onset diabetes after liver and kidney transplantation. Front Endocrinol (Lausanne). 14:1091843. DOI: 10.3389/fendo.2023.1091843. PMID: 36843576. PMCID: PMC9944581.
Article24. Kim JM, Hwang S, Lee KW, Lee JG, Ryu JH, Kim BW, et al. 2020; New-onset diabetes after adult liver transplantation in the Korean Organ Transplantation Registry (KOTRY) study. Hepatobiliary Surg Nutr. 9:425–439. DOI: 10.21037/hbsn.2019.10.29. PMID: 32832494. PMCID: PMC7423540.
Article25. Rostambeigi N, Lanza IR, Dzeja PP, Deeds MC, Irving BA, Reddi HV, et al. 2011; Unique cellular and mitochondrial defects mediate FK506-induced islet beta-cell dysfunction. Transplantation. 91:615–623. DOI: 10.1097/TP.0b013e3182094a33. PMID: 21200364. PMCID: PMC3339767.
Article26. Heisel O, Heisel R, Balshaw R, Keown P. 2004; New onset diabetes mellitus in patients receiving calcineurin inhibitors: a systematic review and meta-analysis. Am J Transplant. 4:583–595. DOI: 10.1046/j.1600-6143.2003.00372.x. PMID: 15023151.
Article27. Pageaux GP, Calmus Y, Boillot O, Ducerf C, Vanlemmens C, Boudjema K, et al. 2004; Steroid withdrawal at day 14 after liver transplantation: a double-blind, placebo-controlled study. Liver Transpl. 10:1454–1460. DOI: 10.1002/lt.20291. PMID: 15558584.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Safety and efficacy of early corticosteroid withdrawal in liver transplant recipients: new-onset diabetes after liver transplantation randomized clinical trial
- Relationship between Uncertainty, Self Efficacy, Social Support, and Self-Care Performance in Liver Transplant Recipients
- Immunizations in adult liver transplant candidates and recipients
- Phase 1/2 donor antigen specific regulatory T cell-based cell therapy clinical trial to induce operational tolerance in living donor liver transplant patients
- Safety and metabolic advantages of steroid withdrawal after 6-months post-transplant in de novo kidney transplantation: 1-year prospective cohort study