Clin Endosc.
2024 May;57(3):384-392. 10.5946/ce.2023.139.
Clinical utility of endoscopic ultrasound-guided tissue acquisition for comprehensive genomic profiling of patients with biliary tract cancer, especially with intrahepatic cholangiocarcinoma
- Affiliations
-
- 1Department of Gastroenterology, Aichi Cancer Center Hospital, Nagoya, Japan
- KMID: 2555900
- DOI: http://doi.org/10.5946/ce.2023.139
Abstract
- Background/Aims
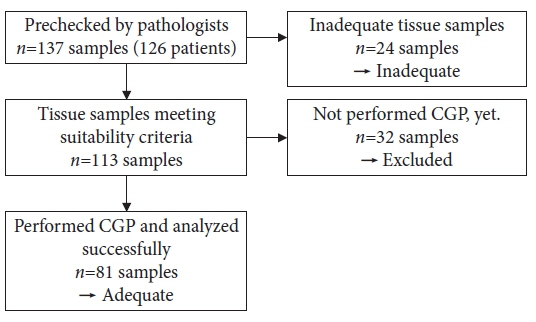
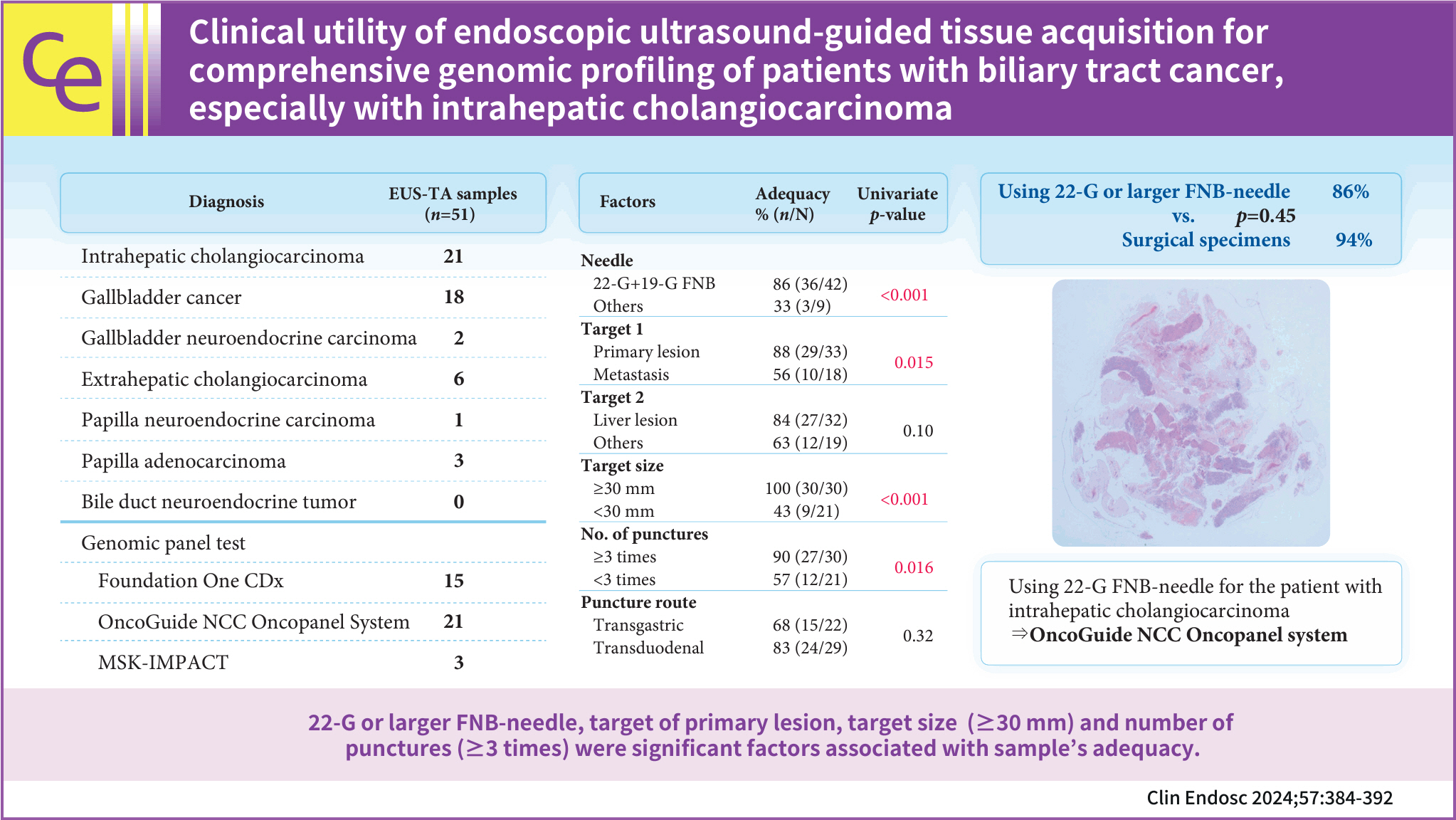
Endoscopic ultrasound-guided tissue acquisition (EUS-TA) is a standard diagnostic method for biliary tract cancer (BTC), and samples obtained in this manner may be used for comprehensive genomic profiling (CGP). This study evaluated the utility of EUS-TA for CGP in a clinical setting and determined the factors associated with the adequacy of CGP in patients with BTC.
Methods
CGP was attempted for 105 samples from 94 patients with BTC at the Aichi Cancer Center, Japan, from October 2019 to April 2022.
Results
Overall, 77.1% (81/105) of the samples were adequate for CGP. For 22-G or 19-G fine-needle biopsy (FNB), the sample adequacy was 85.7% (36/42), which was similar to that of surgical specimens (94%, p=0.45). Univariate analysis revealed that 22-G or larger FNB needle usage (86%, p=0.003), the target primary lesions (88%, p=0.015), a target size ≥30 mm (100%, p=0.0013), and number of punctures (90%, p=0.016) were significantly positively associated with CGP sample adequacy.
Conclusions
EUS-TA is useful for CGP tissue sampling in patients with BTC. In particular, the use of 22-G or larger FNB needles may allow for specimen adequacy comparable to that of surgical specimens.
Keyword
Figure
Cited by 1 articles
-
Is genomic analysis possible in a tissue acquired via endoscopic ultrasound-guided fine-needle biopsy in cholangiocarcinoma?
Jonghyun Lee, Sung Yong Han
Clin Endosc. 2024;57(3):332-334. doi: 10.5946/ce.2024.035.
Reference
-
1. Foundation for Promotion of Cancer Research. Cancer Statistics in Japan 2022. National Cancer Center;2022. [cited 2022 Dec 28]. Available from: https://ganjoho.jp/public/qa_links/report/statistics/2022_en.html.2. Miyakawa S, Ishihara S, Horiguchi A, et al. Biliary tract cancer treatment: 5,584 results from the Biliary Tract Cancer Statistics Registry from 1998 to 2004 in Japan. J Hepatobiliary Pancreat Surg. 2009; 16:1–7.
Article3. Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010; 362:1273–1281.
Article4. Ioka T, Kanai M, Kobayashi S, et al. Randomized phase III study of gemcitabine, cisplatin plus S-1 versus gemcitabine, cisplatin for advanced biliary tract cancer (KHBO1401-MITSUBA). J Hepatobiliary Pancreat Sci. 2023; 30:102–110.
Article5. Oh DY, He AR, Qin S, et al. A phase 3 randomized, double-blind, placebo-controlled study of durvalumab in combination with gemcitabine plus cisplatin (GemCis) in patients (pts) with advanced biliary tract cancer (BTC): TOPAZ-1. J Clin Oncol. 2022. 40(4_suppl):378.
Article6. Lamarca A, Palmer DH, Wasan HS, et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021; 22:690–701.
Article7. Yoo C, Kim KP, Jeong JH, et al. Liposomal irinotecan plus fluorouracil and leucovorin versus fluorouracil and leucovorin for metastatic biliary tract cancer after progression on gemcitabine plus cisplatin (NIFTY): a multicentre, open-label, randomised, phase 2b study. Lancet Oncol. 2021; 22:1560–1572.
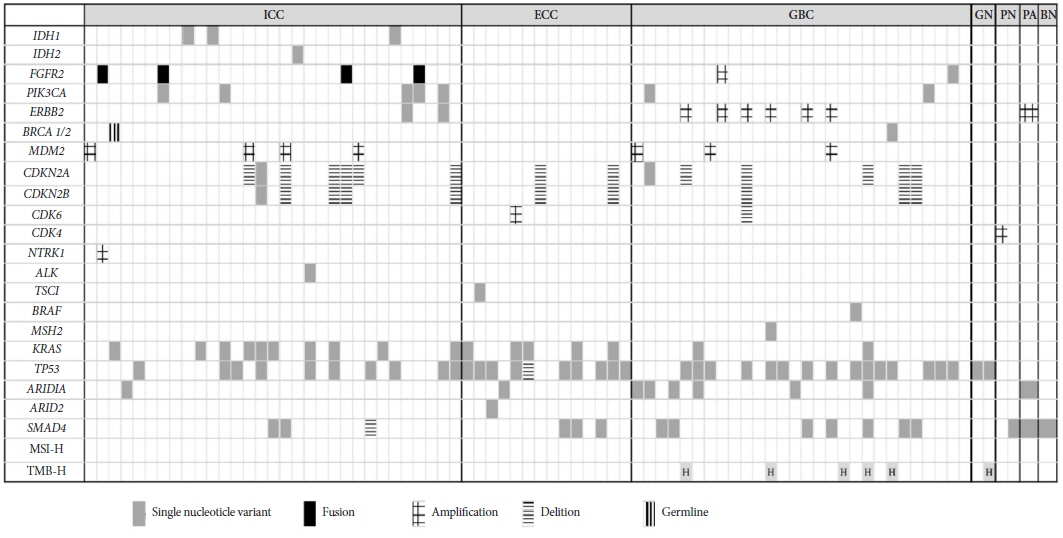
Article8. Nakamura H, Arai Y, Totoki Y, et al. Genomic spectra of biliary tract cancer. Nat Genet. 2015; 47:1003–1010.
Article9. Sadeghi A, Mohamadnejad M, Islami F, et al. Diagnostic yield of EUS-guided FNA for malignant biliary stricture: a systematic review and meta-analysis. Gastrointest Endosc. 2016; 83:290–298.
Article10. Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010; 71:446–454.
Article11. Navaneethan U, Njei B, Lourdusamy V, et al. Comparative effectiveness of biliary brush cytology and intraductal biopsy for detection of malignant biliary strictures: a systematic review and meta-analysis. Gastrointest Endosc. 2015; 81:168–176.
Article12. Rader AE, Avery A, Wait CL, et al. Fine-needle aspiration biopsy diagnosis of gastrointestinal stromal tumors using morphology, immunocytochemistry, and mutational analysis of c-kit. Cancer. 2001; 93:269–275.
Article13. Kuwatani M, Sakamoto N. Evolution and a promising role of EUS-FNA in gene and future analyses. Endosc Ultrasound. 2020; 9:151–153.
Article14. Hirata K, Kuwatani M, Suda G, et al. A novel approach for the genetic analysis of biliary tract cancer specimens obtained through endoscopic ultrasound-guided fine needle aspiration using targeted amplicon sequencing. Clin Transl Gastroenterol. 2019; 10:e00022.
Article15. Ikeda G, Hijioka S, Nagashio Y, et al. Fine-needle biopsy with 19G needle is effective in combination with endoscopic ultrasound-guided tissue acquisition for genomic profiling of unresectable pancreatic cancer. Dig Endosc. 2023; 35:124–133.
Article16. Takahashi K, Yasuda I, Hanaoka T, et al. Comparison of histological sample volumes among various endoscopic ultrasound-guided biopsy needles. J Clin Med. 2021; 10:3560.
Article17. Boscoe AN, Rolland C, Kelley RK. Frequency and prognostic significance of isocitrate dehydrogenase 1 mutations in cholangiocarcinoma: a systematic literature review. J Gastrointest Oncol. 2019; 10:751–765.
Article18. Maruki Y, Morizane C, Arai Y, et al. Molecular detection and clinicopathological characteristics of advanced/recurrent biliary tract carcinomas harboring the FGFR2 rearrangements: a prospective observational study (PRELUDE Study). J Gastroenterol. 2021; 56:250–260.
Article19. Roa I, de Toro G, Schalper K, et al. Overexpression of the HER2/neu gene: a new therapeutic possibility for patients with advanced gallbladder cancer. Gastrointest Cancer Res. 2014; 7:42–48.20. Israel MA, Danziger N, McGregor KA, et al. Comparative genomic analysis of intrahepatic cholangiocarcinoma: biopsy type, ancestry, and testing patterns. Oncologist. 2021; 26:787–796.
Article21. Berchuck JE, Facchinetti F, DiToro DF, et al. The clinical landscape of cell-free DNA alterations in 1671 patients with advanced biliary tract cancer. Ann Oncol. 2022; 33:1269–1283.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical utility of endoscopic ultrasound-guided tissue acquisition for comprehensive genomic profiling of pancreatic cancer
- Fine-Needle Biopsy: Should This Be the First Choice in Endoscopic Ultrasound-Guided Tissue Acquisition?
- Comprehensive management of cholangiocarcinoma: Part I. Diagnosis
- Endoscopic Ultrasonography in the Evaluation of Indeterminate Biliary Strictures
- Endoscopic Ultrasound-guided Radio-frequency Ablation for Pancreatic Cancer