Clin Endosc.
2024 May;57(3):375-383. 10.5946/ce.2023.035.
A novel fully covered metal stent for unresectable malignant distal biliary obstruction: results of a multicenter prospective study
- Affiliations
-
- 1Division of Gastroenterology, Department of Internal Medicine, Kobe University Graduate School of Medicine, Kobe, Japan
- 2Department of Gastroenterology and Hepatology, Osaka Saiseikai Nakatsu Hospital, Osaka, Japan
- 3Department of Gastroenterology, Akashi Medical Center, Akashi, Japan
- 4Department of Gastroenterology, Kita-Harima Medical Center, Ono, Japan
- 5Department of Gastroenterology, Kobe Medical Center, Kobe, Japan
- 6Department of Gastroenterology, Kakogawa Central City Hospital, Kakogawa, Japan
- 7Division of Gastroenterology, Konan Medical Center, Kobe, Japan
- 8Department of Gastroenterology, Hyogo Cancer Center, Akashi, Japan
- 9Department of Gastroenterology, Takatsuki General Hospital, Takatsuki, Japan
- 10Department of Internal Medicine, Shiso Municipal Hospital, Shiso, Japan
- 11Department of Gastroenterology, Kobe Red Cross Hospital, Kobe, Japan
- 12Division of Gastroenterology and Hepatobiliary and Pancreatic Diseases, Department of Internal Medicine, Hyogo Medical University, Nishinomiya, Japan
- KMID: 2555899
- DOI: http://doi.org/10.5946/ce.2023.035
Abstract
- Background/Aims
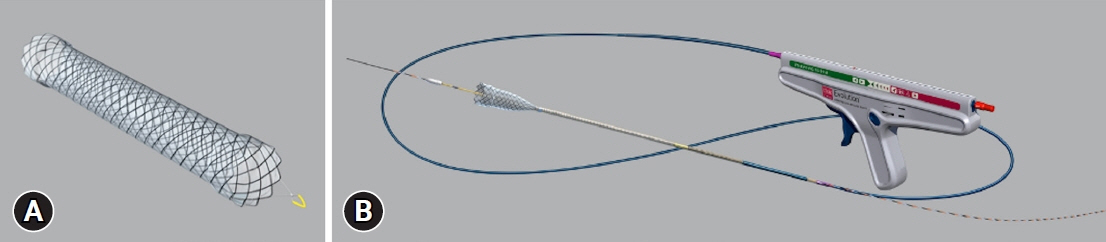
Endoscopic self-expandable metal stent (SEMS) placement is currently the standard technique for treating unresectable malignant distal biliary obstructions (MDBO). Therefore, covered SEMS with longer stent patency and fewer migrations are required. This study aimed to assess the clinical performance of a novel, fully covered SEMS for unresectable MDBO.
Methods
This was a multicenter single-arm prospective study. The primary outcome was a non-obstruction rate at 6 months. The secondary outcomes were overall survival (OS), recurrent biliary obstruction (RBO), time to RBO (TRBO), technical and clinical success, and adverse events.
Results
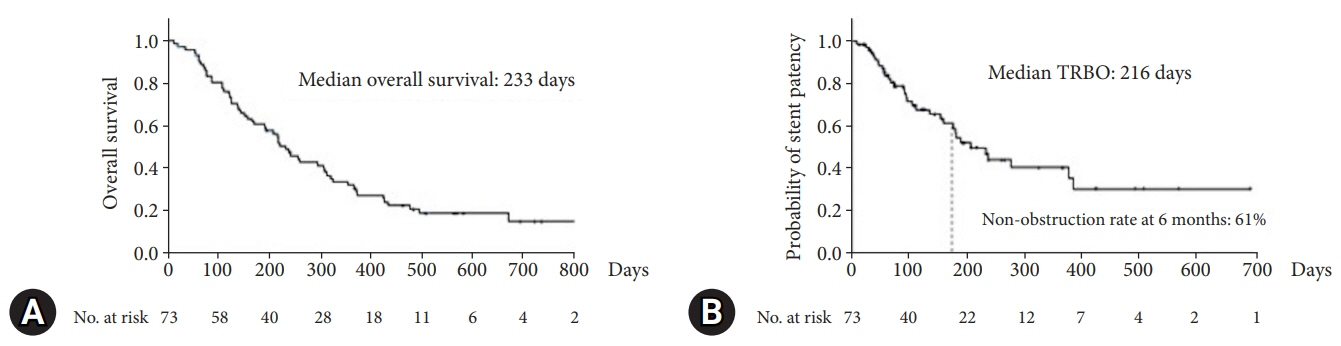
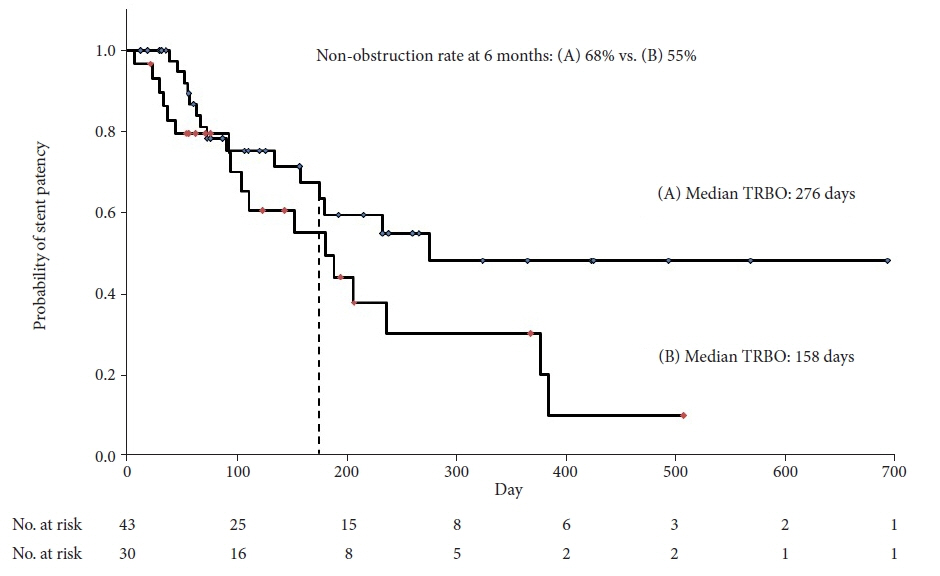
A total of 73 patients were enrolled in this study. The non-obstruction rate at 6 months was 61%. The median OS and TRBO were 233 and 216 days, respectively. The technical and clinical success rates were 100% and 97%, respectively. Furthermore, the rate of occurrence of RBO and adverse events was 49% and 21%, respectively. The length of bile duct stenosis (<2.2 cm) was the only significant risk factor for stent migration.
Conclusions
The non-obstruction rate of a novel fully covered SEMS for MDBO is comparable to that reported earlier but shorter than expected. Short bile duct stenosis is a significant risk factor for stent migration.
Figure
Reference
-
1. Nakai Y, Isayama H, Wang HP, et al. International consensus statements for endoscopic management of distal biliary stricture. J Gastroenterol Hepatol. 2020; 35:967–979.
Article2. Davids PH, Groen AK, Rauws EA, et al. Randomised trial of self-expanding metal stents versus polyethylene stents for distal malignant biliary obstruction. Lancet. 1992; 340:1488–1492.
Article3. Tringali A, Hassan C, Rota M, et al. Covered vs. uncovered self-expandable metal stents for malignant distal biliary strictures: a systematic review and meta-analysis. Endoscopy. 2018; 50:631–641.
Article4. Isayama H, Komatsu Y, Tsujino T, et al. A prospective randomised study of “covered” versus “uncovered” diamond stents for the management of distal malignant biliary obstruction. Gut. 2004; 53:729–734.
Article5. Kitano M, Yamashita Y, Tanaka K, et al. Covered self-expandable metal stents with an anti-migration system improve patency duration without increased complications compared with uncovered stents for distal biliary obstruction caused by pancreatic carcinoma: a randomized multicenter trial. Am J Gastroenterol. 2013; 108:1713–1722.
Article6. Nakai Y, Isayama H, Kogure H, et al. Risk factors for covered metallic stent migration in patients with distal malignant biliary obstruction due to pancreatic cancer. J Gastroenterol Hepatol. 2014; 29:1744–1749.
Article7. Lawrence C, Nieto J, Parsons WG, et al. A newly designed uncovered biliary stent for palliation of malignant obstruction: results of a prospective study. BMC Gastroenterol. 2020; 20:184.
Article8. Isayama H, Hamada T, Yasuda I, et al. TOKYO criteria 2014 for transpapillary biliary stenting. Dig Endosc. 2015; 27:259–264.9. Kahaleh M, Talreja JP, Loren DE, et al. Evaluation of a fully covered self-expanding metal stent with flared ends in malignant biliary obstruction: a multicenter study. J Clin Gastroenterol. 2013; 47:e96–e100.10. Telford JJ, Carr-Locke DL, Baron TH, et al. A randomized trial comparing uncovered and partially covered self-expandable metal stents in the palliation of distal malignant biliary obstruction. Gastrointest Endosc. 2010; 72:907–914.
Article11. Lee JH, Krishna SG, Singh A, et al. Comparison of the utility of covered metal stents versus uncovered metal stents in the management of malignant biliary strictures in 749 patients. Gastrointest Endosc. 2013; 78:312–324.
Article12. Yang MJ, Kim JH, Yoo BM, et al. Partially covered versus uncovered self-expandable nitinol stents with anti-migration properties for the palliation of malignant distal biliary obstruction: a randomized controlled trial. Scand J Gastroenterol. 2015; 50:1490–1499.
Article13. Krokidis M, Fanelli F, Orgera G, et al. Percutaneous treatment of malignant jaundice due to extrahepatic cholangiocarcinoma: covered Viabil stent versus uncovered Wallstents. Cardiovasc Intervent Radiol. 2010; 33:97–106.
Article14. Krokidis M, Fanelli F, Orgera G, et al. Percutaneous palliation of pancreatic head cancer: randomized comparison of ePTFE/FEP-covered versus uncovered nitinol biliary stents. Cardiovasc Intervent Radiol. 2011; 34:352–361.
Article15. Knyrim K, Wagner HJ, Pausch J, et al. A prospective, randomized, controlled trial of metal stents for malignant obstruction of the common bile duct. Endoscopy. 1993; 25:207–212.
Article16. Prat F, Chapat O, Ducot B, et al. A randomized trial of endoscopic drainage methods for inoperable malignant strictures of the common bile duct. Gastrointest Endosc. 1998; 47:1–7.17. Yamao K, Takenaka M, Ogura T, et al. Utility and safety of a novel fully covered metal stent in unresectable distal malignant biliary obstruction. Dig Dis Sci. 2020; 65:3702–3709.
Article18. Marui S, Uza N, Yamazaki H, et al. Utility of laser-cut covered self-expandable metal stents for unresectable malignant distal biliary obstruction: a single-center experience. Endoscopy. 2020; 52:664–668.
Article19. Kogure H, Ryozawa S, Maetani I, et al. A prospective multicenter study of a fully covered metal stent in patients with distal malignant biliary obstruction: WATCH-2 study. Dig Dis Sci. 2018; 63:2466–2473.20. Park SB, Kim HW, Kang DH, et al. Metallic or plastic stent for bile duct obstruction in ampullary cancer? Dig Dis Sci. 2012; 57:786–790.21. Isayama H, Nakai Y, Toyokawa Y, et al. Measurement of radial and axial forces of biliary self-expandable metallic stents. Gastrointest Endosc. 2009; 70:37–44.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Endoscopic Stent Placement in the Palliation of Malignant Biliary Obstruction
- Proximal Stent Migration of Fully Covered Self-Expandable Metal Stent following Side-by-Side Deployment in the Patient with Klatskin Tumor: A Case Report
- A Comparison of Covered Expandable Metal Stent and Uncovered Expandable Metal Stent for the Management of Distal Malignant Biliary Obstruction
- Simultaneous Duodenal Metal Stent Placement and EUS-Guided Choledochoduodenostomy for Unresectable Pancreatic Cancer
- A Comparative Study on the Efficacy of Covered Metal Stent and Plastic Stent in Malignant Biliary Obstruction