Clin Endosc.
2024 May;57(3):364-374. 10.5946/ce.2023.095.
Comparison of 19-gauge conventional and Franseen needles for the diagnosis of lymphadenopathy and classification of malignant lymphoma using endoscopic ultrasound fine-needle aspiration
- Affiliations
-
- 1Department of Gastroenterology, Gifu Municipal Hospital, Gifu, Japan
- 2Department of Gastroenterological Endoscopy, Kanazawa Medical University, Ishikawa, Japan
- 3Department of Pathology and Translational Research, Gifu University Graduate School of Medicine, Gifu, Japan
- 4Department of Diagnostic Pathology, Gifu Municipal Hospital, Gifu, Japan
- 5Department of Hematology, Gifu Municipal Hospital, Gifu, Japan
- 6First Department of Internal Medicine, Gifu Univeristy Hospital, Gifu, Japan
- KMID: 2555898
- DOI: http://doi.org/10.5946/ce.2023.095
Abstract
- Background/Aims
Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) using a 19-gauge needle is an efficient sampling method for the diagnosis of lymphadenopathy. This study compared 19-gauge conventional and Franseen needles for the diagnosis of lymphadenopathy and classification of malignant lymphoma (ML).
Methods
Patient characteristics, number of needle passes, puncture route, sensitivity, specificity, and accuracy of cytology/histology for lymphadenopathy were analyzed in patients diagnosed with lymphadenopathy by EUS-FNA using conventional or Franseen needles.
Results
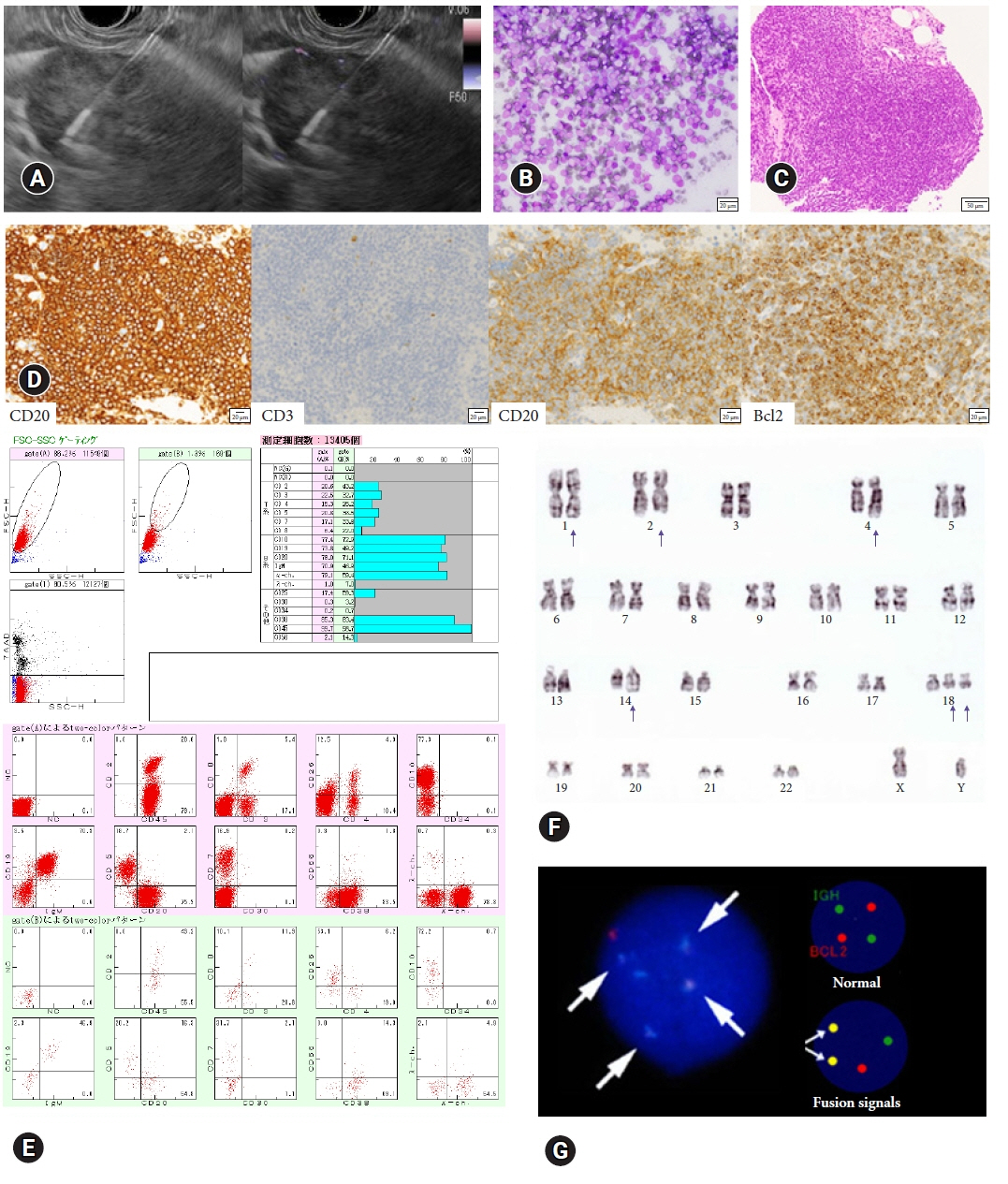
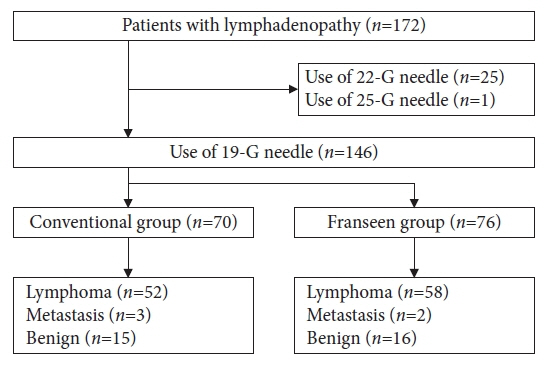
Between 2012 and 2022, 146 patients met the inclusion criteria (conventional [n=70] and Franseen [n=76]). The median number of needle passes was significantly lower in the conventional group than in the Franseen group (3 [1–6] vs. 4 [1–6], p=0.023). There were no significant differences in cytological/histological diagnoses between the two groups. For ML, the immunohistochemical evaluation rate, sensitivity of flow cytometry, and cytogenetic assessment were not significantly different in either group. Bleeding as adverse events (AEs) were observed in three patients in the Franseen group.
Conclusions
Both the 19-gauge conventional and Franseen needles showed high accuracy in lymphadenopathy and ML classification. Considering sufficient tissue collection and the avoidance of AEs, the use of 19-gauge conventional needles seems to be a good option for the diagnosis of lymphadenopathy.
Keyword
Figure
Cited by 1 articles
-
Choosing needles wisely: 19-G conventional vs. Franseen needles in endoscopic ultrasound-guided fine-needle aspiration for malignant lymphoma diagnosis and classification
Kajornvit Raghareutai, Worapoth Yingyongthawat, Nonthalee Pausawasdi
Clin Endosc. 2024;57(4):473-475. doi: 10.5946/ce.2024.129.
Reference
-
1. Wani S, Muthusamy VR, Komanduri S. EUS-guided tissue acquisition: an evidence-based approach (with videos). Gastrointest Endosc. 2014; 80:939–959.
Article2. Yasuda I, Goto N, Tsurumi H, et al. Endoscopic ultrasound-guided fine needle aspiration biopsy for diagnosis of lymphoproliferative disorders: feasibility of immunohistological, flow cytometric, and cytogenetic assessments. Am J Gastroenterol. 2012; 107:397–404.
Article3. Itoi T, Tsuchiya T, Itokawa F, et al. Histological diagnosis by EUS-guided fine-needle aspiration biopsy in pancreatic solid masses without on-site cytopathologist: a single-center experience. Dig Endosc. 2011; 23 Suppl 1:34–38.
Article4. Bang JY, Hawes R, Varadarajulu S. A meta-analysis comparing ProCore and standard fine-needle aspiration needles for endoscopic ultrasound-guided tissue acquisition. Endoscopy. 2016; 48:339–349.
Article5. Yasuda I, Tsurumi H, Omar S, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy for lymphadenopathy of unknown origin. Endoscopy. 2006; 38:919–924.
Article6. Mukai S, Itoi T, Yamaguchi H, et al. A retrospective histological comparison of EUS-guided fine-needle biopsy using a novel franseen needle and a conventional end-cut type needle. Endosc Ultrasound. 2019; 8:50–57.
Article7. Sugimoto M, Irie H, Takagi T, et al. Efficacy of EUS-guided FNB using a Franseen needle for tissue acquisition and microsatellite instability evaluation in unresectable pancreatic lesions. BMC Cancer. 2020; 20:1094.
Article8. de Moura DT, McCarty TR, Jirapinyo P, et al. EUS-guided fine-needle biopsy sampling versus FNA in the diagnosis of subepithelial lesions: a large multicenter study. Gastrointest Endosc. 2020; 92:108–119.
Article9. Itonaga M, Yasukawa S, Fukutake N, et al. Comparison of 22-gauge standard and Franseen needles in EUS-guided tissue acquisition for diagnosing solid pancreatic lesions: a multicenter randomized controlled trial. Gastrointest Endosc. 2022; 96:57–66.
Article10. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016; 127:2375–2390.
Article11. Facciorusso A, Crinò SF, Muscatiello N, et al. Endoscopic ultrasound fine-needle biopsy versus fine-needle aspiration for tissue sampling of abdominal lymph nodes: a propensity score matched multicenter comparative study. Cancers (Basel). 2021; 13:4298.
Article12. Hedenström P, Chatzikyriakos V, Shams R, et al. High sensitivity of endoscopic ultrasound-guided fine-needle aspiration and endoscopic ultrasound-guided fine-needle biopsy in lymphadenopathy caused by metastatic disease: a prospective comparative study. Clin Endosc. 2021; 54:722–729.
Article13. de Moura DT, McCarty TR, Jirapinyo P, et al. Endoscopic ultrasound fine-needle aspiration versus fine-needle biopsy for lymph node diagnosis: a large multicenter comparative analysis. Clin Endosc. 2020; 53:600–610.
Article14. Farinha P, Gascoyne RD. Molecular pathogenesis of mucosa-associated lymphoid tissue lymphoma. J Clin Oncol. 2005; 23:6370–6378.
Article15. Nadel B, Marculescu R, Le T, et al. Novel insights into the mechanism of t(14;18)(q32;q21) translocation in follicular lymphoma. Leuk Lymphoma. 2001; 42:1181–1194.
Article16. Le Gouill S, Talmant P, Touzeau C, et al. The clinical presentation and prognosis of diffuse large B-cell lymphoma with t(14;18) and 8q24/c-MYC rearrangement. Haematologica. 2007; 92:1335–1342.
Article17. Camacho FI, García JF, Cigudosa JC, et al. Aberrant Bcl6 protein expression in mantle cell lymphoma. Am J Surg Pathol. 2004; 28:1051–1056.
Article18. Yoshida K, Iwashita T, Uemura S, et al. Efficacy of contrast-enhanced EUS for lymphadenopathy: a prospective multicenter pilot study (with videos). Gastrointest Endosc. 2019; 90:242–250.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- 22-gauge Co-Cr versus stainless-steel Franseen needles for endoscopic ultrasound-guided tissue acquisition in patients with solid pancreatic lesions
- Procore and Flexible 19 Gauge Needle Can Replace Trucut Biopsy Needle?
- Choosing needles wisely: 19-G conventional vs. Franseen needles in endoscopic ultrasound-guided fine-needle aspiration for malignant lymphoma diagnosis and classification
- Comparison of Endoscopic Ultrasound-Guided Tissue Acquisition Using a 20-Gauge Menghini Needle with a Lateral Forward Bevel and a 22-Gauge Franseen Needle: A Single-Center Large Cohort Study
- Performance Characteristics of a New Flexible Nitinol 19-Gauge Endoscopic Ultrasound-Guided Fine Needle Aspiration Needle