Clin Endosc.
2024 Mar;57(2):237-245. 10.5946/ce.2023.011.
22-gauge Co-Cr versus stainless-steel Franseen needles for endoscopic ultrasound-guided tissue acquisition in patients with solid pancreatic lesions
- Affiliations
-
- 1Department of Gastroenterology, Saitama Medical University International Medical Center, Saitama, Japan
- KMID: 2553761
- DOI: http://doi.org/10.5946/ce.2023.011
Abstract
- Background/Aims
Endoscopic ultrasound-guided tissue acquisition (EUS-TA) using Franseen needles is reportedly useful for its high diagnostic yield. This study compared the diagnostic yield and puncturing ability of EUS-TA using 22-gauge cobalt-chromium (CO-Cr) needles with those of stainless-steel Franseen needles in patients with solid pancreatic lesions.
Methods
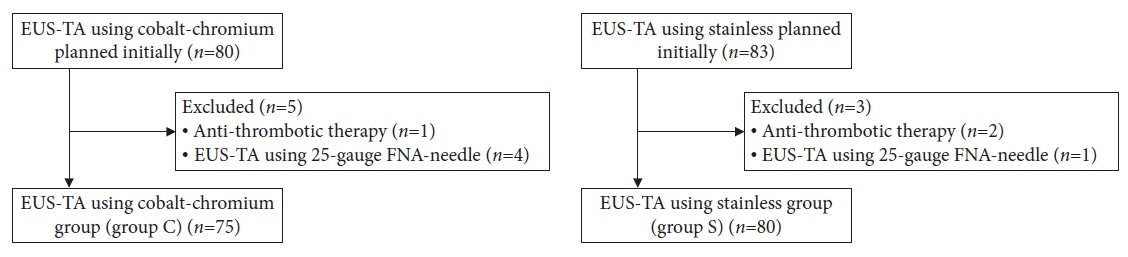
Outcomes were compared between the 22-gauge Co-Cr Franseen needle (December 2019 to November 2020; group C) and stainless-steel needle (November 2020 to May 2022; group S).
Results
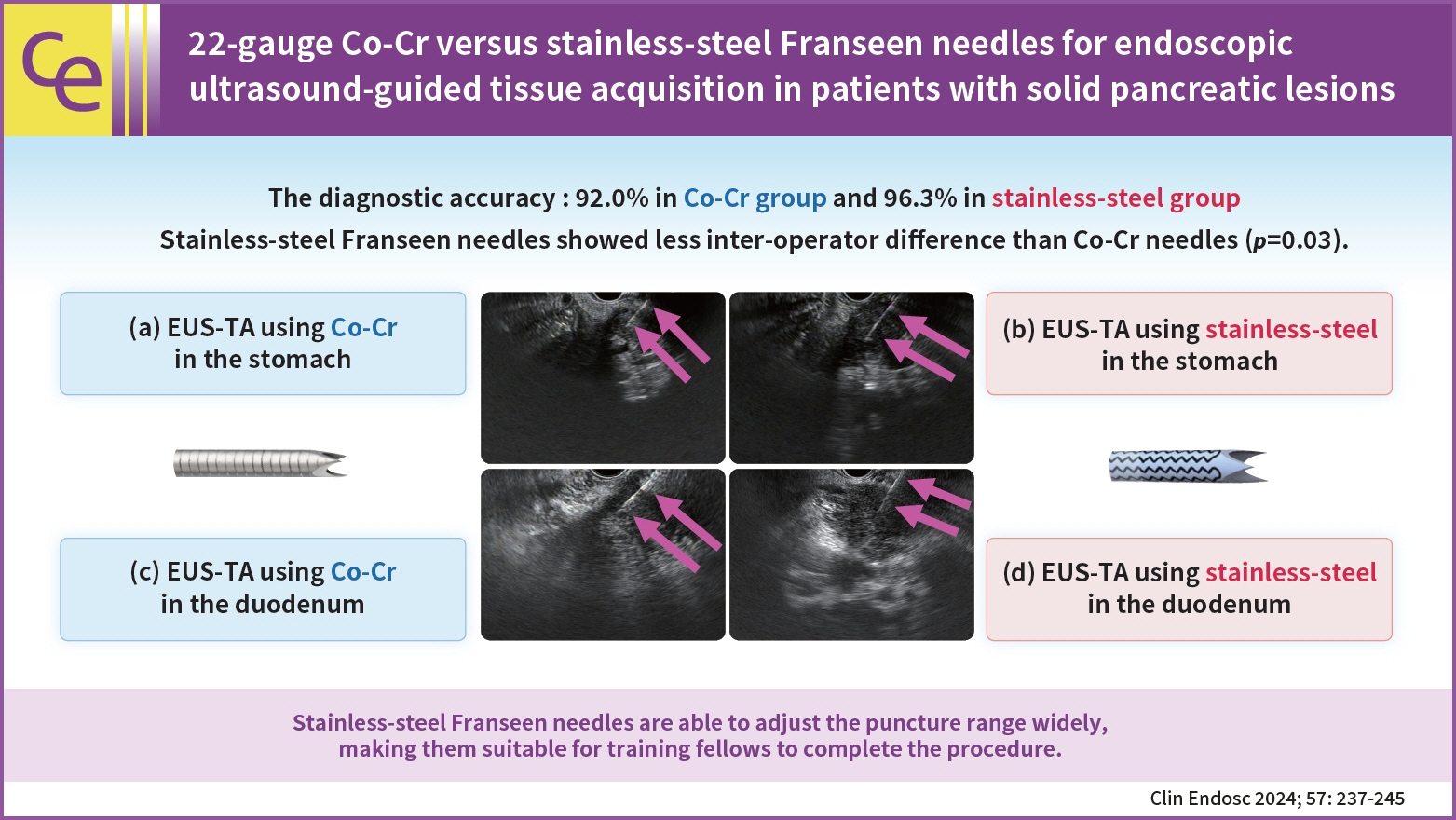
A total of 155 patients (group C, 75; group S, 80) were eligible. The diagnostic accuracy was 92.0% in group C and 96.3% in group S with no significant intergroup differences (p=0.32). The rate of change in the operator (from training fellows to experts) was 20.0% (15/75) in group C and 7.5% (6/80) in group S. Stainless-steel Franseen needles showed less inter-operator difference than Co-Cr needles (p=0.03).
Conclusions
Both Co-Cr and stainless-steel Franseen needles showed high diagnostic ability. Stainless-steel Franseen needles are soft and flexible; therefore, the range of puncture angles can be widely adjusted, making them suitable for training fellows to complete the procedure.
Keyword
Figure
Reference
-
1. Iglesias-Garcia J, Dominguez-Munoz JE, Abdulkader I, et al. Influence of on-site cytopathology evaluation on the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) of solid pancreatic masses. Am J Gastroenterol. 2011; 106:1705–1710.2. Vilmann P, Săftoiu A, Hollerbach S, et al. Multicenter randomized controlled trial comparing the performance of 22 gauge versus 25 gauge EUS-FNA needles in solid masses. Scand J Gastroenterol. 2013; 48:877–883.3. Puli SR, Bechtold ML, Buxbaum JL, et al. How good is endoscopic ultrasound-guided fine-needle aspiration in diagnosing the correct etiology for a solid pancreatic mass?: a meta-analysis and systematic review. Pancreas. 2013; 42:20–26.4. Iwashita T, Yasuda I, Doi S, et al. Use of samples from endoscopic ultrasound-guided 19-gauge fine-needle aspiration in diagnosis of autoimmune pancreatitis. Clin Gastroenterol Hepatol. 2012; 10:316–322.5. Bang JY, Hebert-Magee S, Trevino J, et al. Randomized trial comparing the 22-gauge aspiration and 22-gauge biopsy needles for EUS-guided sampling of solid pancreatic mass lesions. Gastrointest Endosc. 2012; 76:321–327.6. Iwashita T, Nakai Y, Samarasena JB, et al. High single-pass diagnostic yield of a new 25-gauge core biopsy needle for EUS-guided FNA biopsy in solid pancreatic lesions. Gastrointest Endosc. 2013; 77:909–915.7. Fujita A, Ryozawa S, Tanisaka Y, et al. Evaluation of endoscopic ultrasound-guided fine-needle biopsy for preoperative pancreatic solid lesions. Scand J Gastroenterol. 2021; 56:188–192.8. Tanisaka Y, Mizuide M, Fujita A, et al. Comparison of endoscopic ultrasound-guided fine-needle aspiration and biopsy device for lymphadenopathy. Gastroenterol Res Pract. 2021; 2021:6640862.9. Itonaga M, Yasukawa S, Fukutake N, et al. Comparison of 22-gauge standard and Franseen needles in EUS-guided tissue acquisition for diagnosing solid pancreatic lesions: a multicenter randomized controlled trial. Gastrointest Endosc. 2022; 96:57–66.10. Kandel P, Nassar A, Gomez V, et al. Comparison of endoscopic ultrasound-guided fine-needle biopsy versus fine-needle aspiration for genomic profiling and DNA yield in pancreatic cancer: a randomized crossover trial. Endoscopy. 2021; 53:376–382.11. Ikeda G, Hijioka S, Nagashio Y, et al. Fine-needle biopsy with 19G needle is effective in combination with endoscopic ultrasound-guided tissue acquisition for genomic profiling of unresectable pancreatic cancer. Dig Endosc. 2023; 35:124–133.12. Bang JY, Hebert-Magee S, Hasan MK, et al. Endoscopic ultrasonography-guided biopsy using a Franseen needle design: initial assessment. Dig Endosc. 2017; 29:338–346.13. Mukai S, Itoi T, Yamaguchi H, et al. A retrospective histological comparison of EUS-guided fine-needle biopsy using a novel franseen needle and a conventional end-cut type needle. Endosc Ultrasound. 2019; 8:50–57.14. Kurita A, Yasukawa S, Zen Y, et al. Comparison of a 22-gauge Franseen-tip needle with a 20-gauge forward-bevel needle for the diagnosis of type 1 autoimmune pancreatitis: a prospective, randomized, controlled, multicenter study (COMPAS study). Gastrointest Endosc. 2020; 91:373–381.15. Ishigaki K, Nakai Y, Sasahira N, et al. A prospective multicenter study of endoscopic ultrasound-guided fine needle biopsy using a 22-gauge Franseen needle for pancreatic solid lesions. J Gastroenterol Hepatol. 2021; 36:2754–2761.16. Nakai Y, Isayama H, Chang KJ, et al. Slow pull versus suction in endoscopic ultrasound-guided fine-needle aspiration of pancreatic solid masses. Dig Dis Sci. 2014; 59:1578–1585.17. Saxena P, El Zein M, Stevens T, et al. Stylet slow-pull versus standard suction for endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic lesions: a multicenter randomized trial. Endoscopy. 2018; 50:497–504.18. Bor R, Vasas B, Fábián A, et al. Slow-pull technique yields better quality smears: prospective comparison of slow-pull and standard suction techniques of endoscopic ultrasound-guided fine-needle aspiration. Scand J Gastroenterol. 2020; 55:1369–1376.19. Matynia AP, Schmidt RL, Barraza G, et al. Impact of rapid on-site evaluation on the adequacy of endoscopic-ultrasound guided fine-needle aspiration of solid pancreatic lesions: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2014; 29:697–705.20. Kouanda A, Mclean R, Faggen A, et al. Telecytology versus in-room cytopathologist for EUS-guided FNA or fine-needle biopsy sampling of solid pancreatic lesions. Gastrointest Endosc. 2023; 97:466–471.21. Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010; 71:446–454.22. Bang JY, Hebert-Magee S, Navaneethan U, et al. Randomized trial comparing the Franseen and Fork-tip needles for EUS-guided fine-needle biopsy sampling of solid pancreatic mass lesions. Gastrointest Endosc. 2018; 87:1432–1438.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Endoscopic Ultrasound-Guided Tissue Acquisition by 22-Gauge Franseen and Standard Needles for Solid Pancreatic Lesions
- Comparison of Endoscopic Ultrasound-Guided Tissue Acquisition Using a 20-Gauge Menghini Needle with a Lateral Forward Bevel and a 22-Gauge Franseen Needle: A Single-Center Large Cohort Study
- Endoscopic ultrasound-guided tissue acquisition: Needle types, technical issues, and sample handling
- Comparison of the Diagnostic Yield of the Standard 22-Gauge Needle and the New 20-Gauge Forward-Bevel Core Biopsy Needle for Endoscopic Ultrasound-Guided Tissue Acquisition from Pancreatic Lesions
- Comparison of 19-gauge conventional and Franseen needles for the diagnosis of lymphadenopathy and classification of malignant lymphoma using endoscopic ultrasound fine-needle aspiration