Clin Endosc.
2024 May;57(3):350-363. 10.5946/ce.2023.144.
Endoscopic resection of gastric gastrointestinal stromal tumor using clip-and-cut endoscopic full-thickness resection: a single-center, retrospective cohort in Korea
- Affiliations
-
- 1Department of Gastroenterology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Department of Internal Medicine, Inje University Ilsan Paik Hospital, Inje University College of Medicine, Goyang, Korea
- 3Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2555897
- DOI: http://doi.org/10.5946/ce.2023.144
Abstract
- Background/Aims
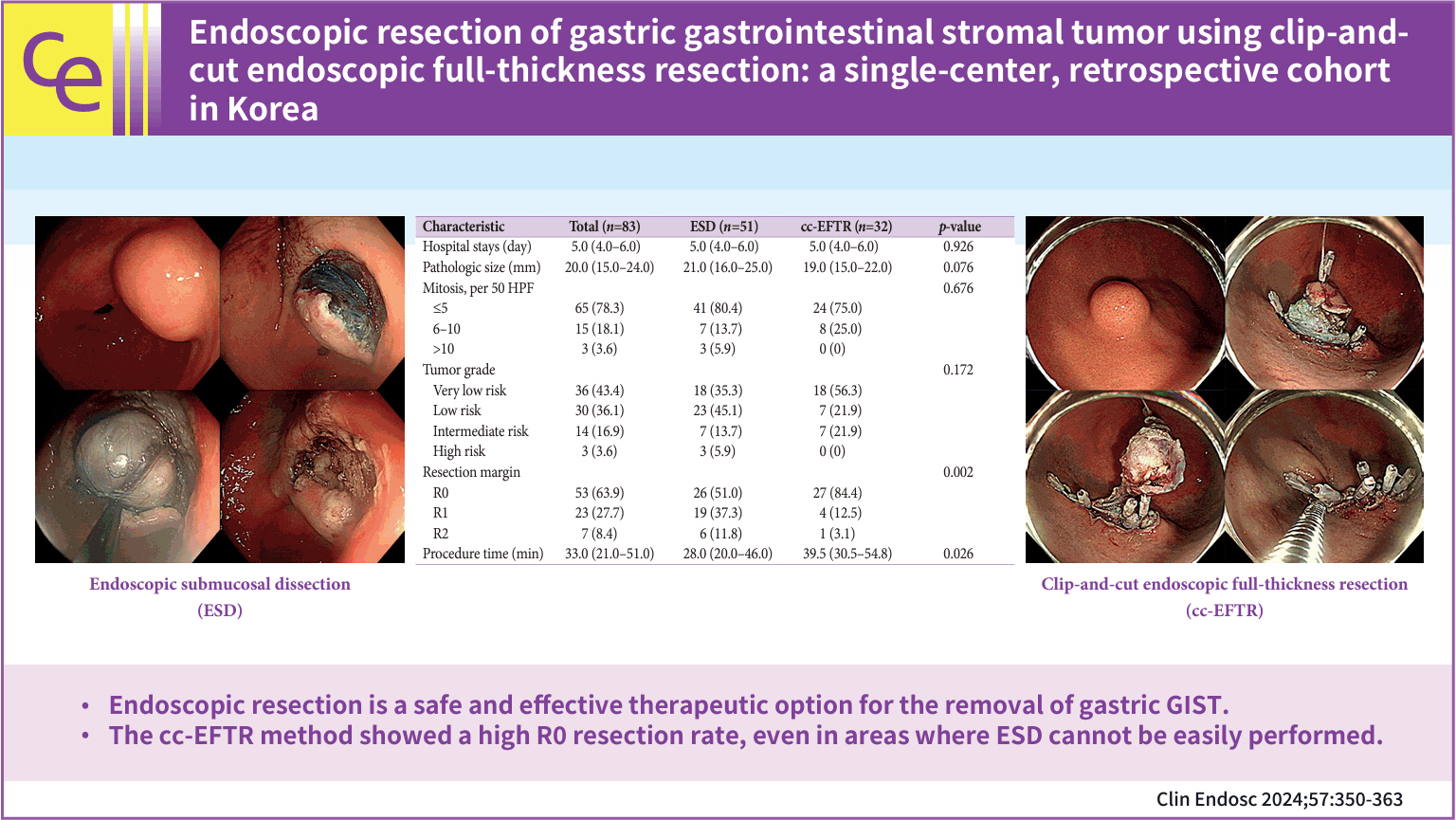
To overcome the technical limitations of classic endoscopic resection for gastric gastrointestinal stromal tumors (GISTs), various methods have been developed. In this study, we examined the role and feasibility of clip-and-cut procedures (clip-and-cut endoscopic full-thickness resection [cc-EFTR]) for gastric GISTs.
Methods
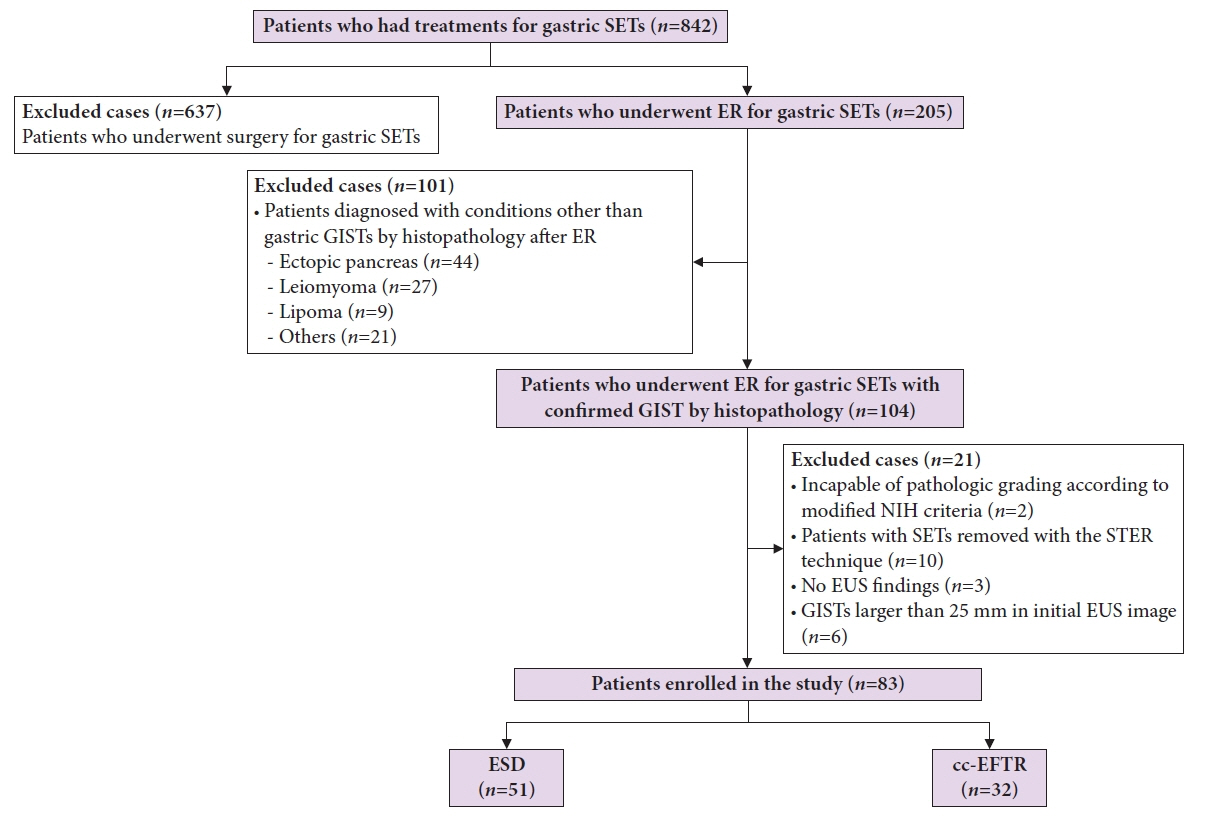
Medical records of 83 patients diagnosed with GISTs after endoscopic resection between 2005 and 2021 were retrospectively reviewed. Moreover, clinical characteristics and outcomes were analyzed.
Results
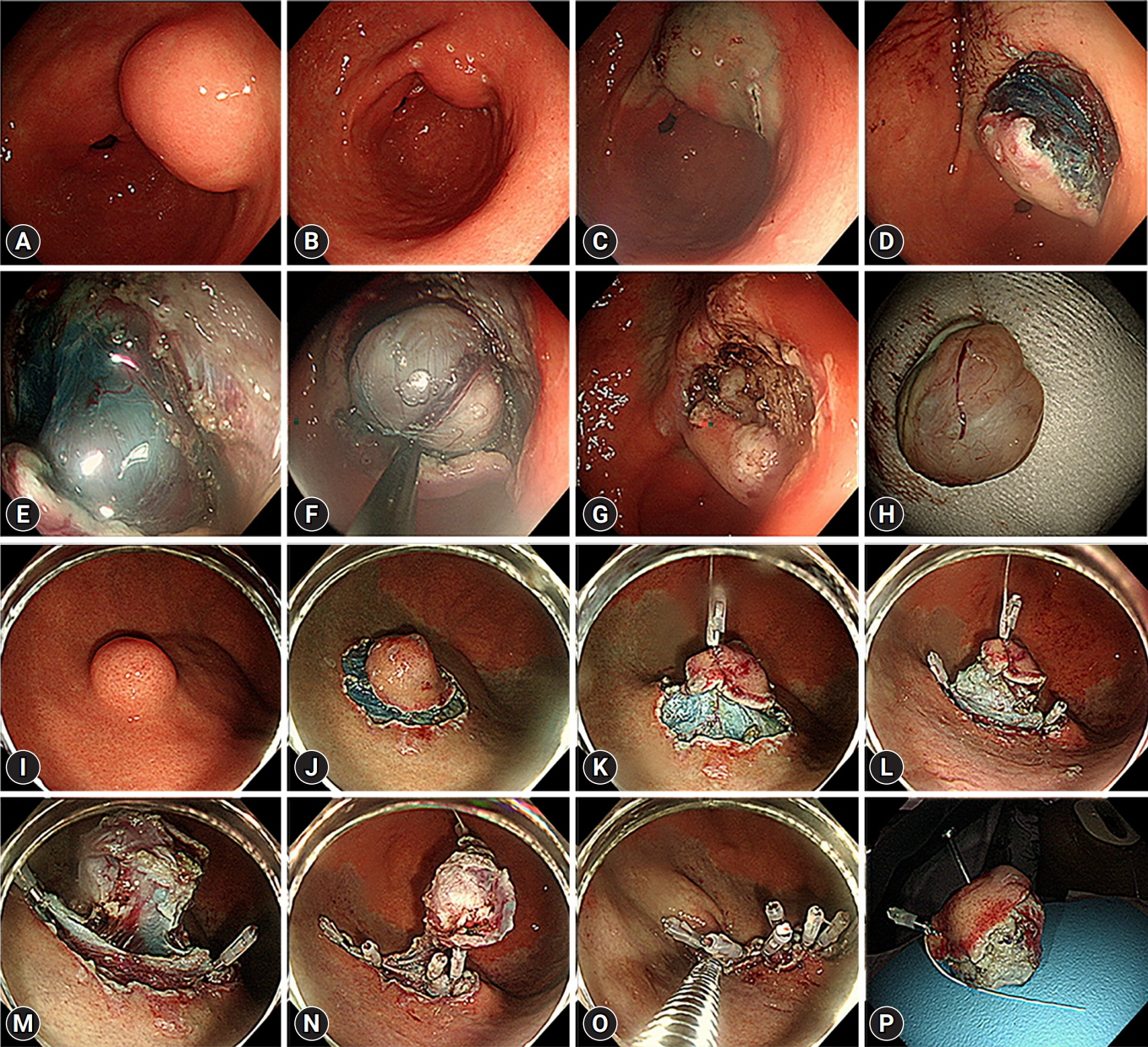
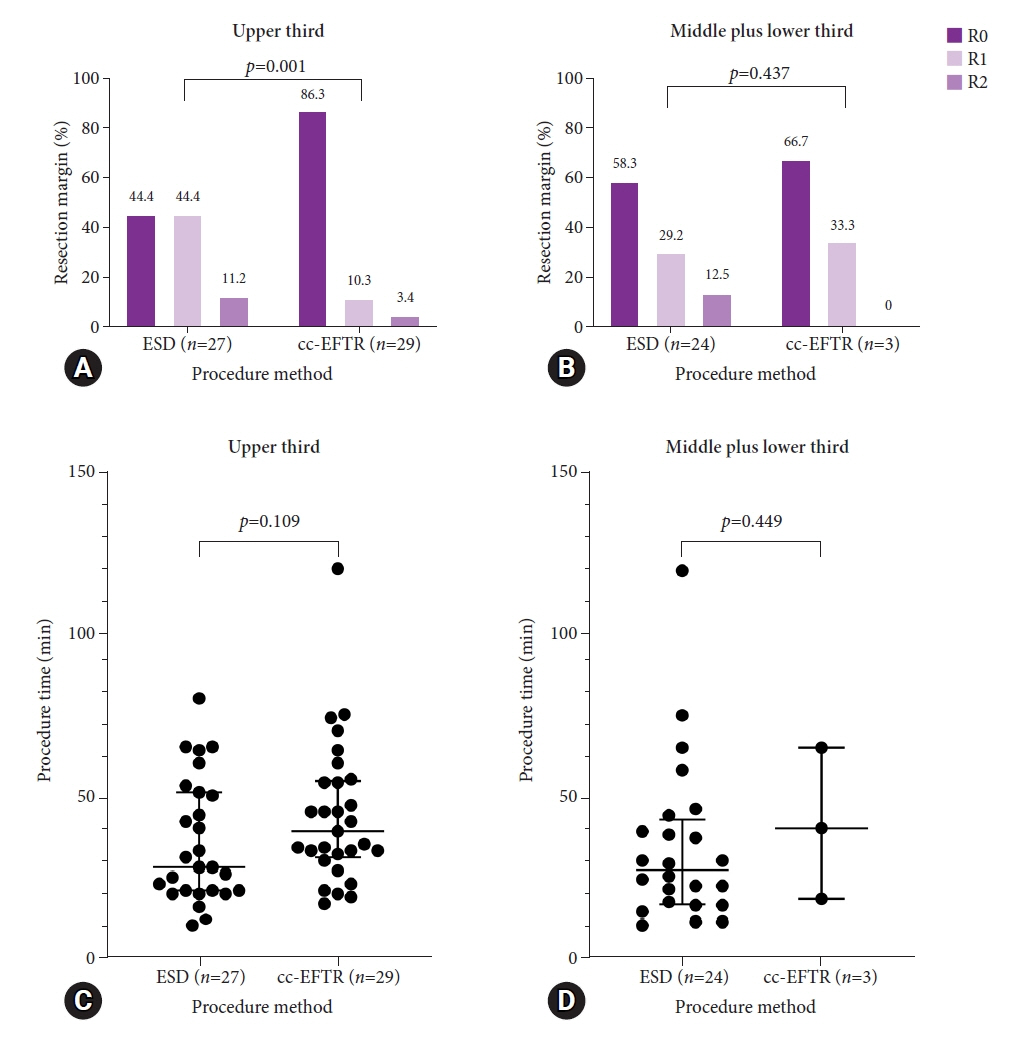
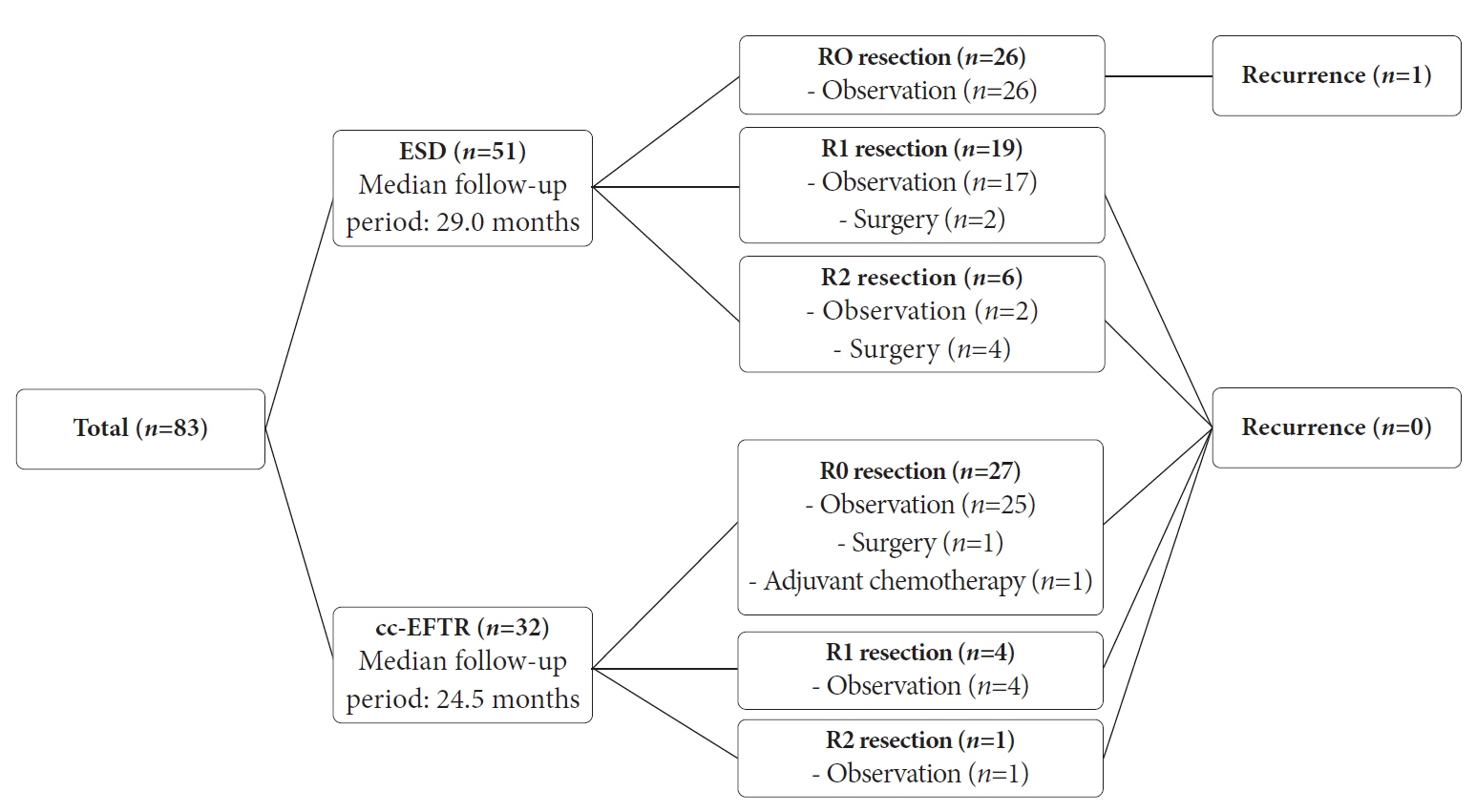
Endoscopic submucosal dissection (ESD) and cc-EFTR were performed in 51 and 32 patients, respectively. The GISTs were detected in the upper third of the stomach for ESD (52.9%) and cc-EFTR (90.6%). Within the cc-EFTR group, a majority of GISTs were located in the deep muscularis propria or serosal layer, accounting for 96.9%, as opposed to those in the ESD group (45.1%). The R0 resection rates were 51.0% and 84.4% in the ESD and cc-EFTR groups, respectively. Seven (8.4%) patients required surgical treatment (six patients underwent ESD and one underwent cc-EFTR,) due to residual tumor (n=5) and post-procedure adverse events (n=2). Patients undergoing R0 or R1 resection did not experience recurrence during a median 14-month follow-up period, except for one patient in the ESD group.
Conclusions
cc-EFTR displayed a high R0 resection rate; therefore, it is a safe and effective therapeutic option for small gastric GISTs.
Keyword
Figure
Cited by 1 articles
-
Endoscopic resection penetrating the muscularis propria for gastric gastrointestinal stromal tumors: advances and challenges
Jin Woong Cho
Clin Endosc. 2024;57(3):329-331. doi: 10.5946/ce.2024.036.
Reference
-
1. Deprez PH, Moons LM, OʼToole D, et al. Endoscopic management of subepithelial lesions including neuroendocrine neoplasms: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022; 54:412–429.
Article2. Lu J, Lu X, Jiao T, et al. Endoscopic management of upper gastrointestinal submucosal tumors arising from muscularis propria. J Clin Gastroenterol. 2014; 48:667–673.
Article3. Cho JW; Korean ESD Study Group. Current guidelines in the management of upper gastrointestinal subepithelial tumors. Clin Endosc. 2016; 49:235–240.
Article4. Yang Z, Gao Y, Fan X, et al. A multivariate prediction model for high malignancy potential gastric GI stromal tumors before endoscopic resection. Gastrointest Endosc. 2020; 91:813–822.
Article5. Parab TM, DeRogatis MJ, Boaz AM, et al. Gastrointestinal stromal tumors: a comprehensive review. J Gastrointest Oncol. 2019; 10:144–154.
Article6. Ko WJ, Cho JY. Current techniques for treating gastrointestinal stromal tumors in the upper gastrointestinal tract. Clin Endosc. 2016; 49:226–228.
Article7. Schmieder M, Henne-Bruns D, Mayer B, et al. Comparison of different risk classification systems in 558 patients with gastrointestinal stromal tumors after R0-resection. Front Pharmacol. 2016; 7:504.
Article8. Demetri GD, von Mehren M, Antonescu CR, et al. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010; 8 Suppl 2(0 2):S1–S41.
Article9. Kim GH, Choi KD, Gong CS, et al. Comparison of the treatment outcomes of endoscopic and surgical resection of GI stromal tumors in the stomach: a propensity score-matched case-control study. Gastrointest Endosc. 2020; 91:527–536.
Article10. Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008; 39:1411–1419.
Article11. Chak A, Canto MI, Rösch T, et al. Endosonographic differentiation of benign and malignant stromal cell tumors. Gastrointest Endosc. 1997; 45:468–473.
Article12. Palazzo L, Landi B, Cellier C, et al. Endosonographic features predictive of benign and malignant gastrointestinal stromal cell tumours. Gut. 2000; 46:88–92.
Article13. Joo MK, Park JJ, Kim H, et al. Endoscopic versus surgical resection of GI stromal tumors in the upper GI tract. Gastrointest Endosc. 2016; 83:318–326.
Article14. McCarter MD, Antonescu CR, Ballman KV, et al. Microscopically positive margins for primary gastrointestinal stromal tumors: analysis of risk factors and tumor recurrence. J Am Coll Surg. 2012; 215:53–60.
Article15. Zhu Y, Xu MD, Xu C, et al. Microscopic positive tumor margin does not increase the rate of recurrence in endoscopic resected gastric mesenchymal tumors compared to negative tumor margin. Surg Endosc. 2020; 34:159–169.
Article16. Chapman BC, McIntosh KE, Jones EL, et al. Postoperative pneumoperitoneum: is it normal or pathologic? J Surg Res. 2015; 197:107–111.
Article17. Li QL, Yao LQ, Zhou PH, et al. Submucosal tumors of the esophagogastric junction originating from the muscularis propria layer: a large study of endoscopic submucosal dissection (with video). Gastrointest Endosc. 2012; 75:1153–1158.
Article18. An W, Sun PB, Gao J, et al. Endoscopic submucosal dissection for gastric gastrointestinal stromal tumors: a retrospective cohort study. Surg Endosc. 2017; 31:4522–4531.
Article19. Białek A, Wiechowska-Kozłowska A, Pertkiewicz J, et al. Endoscopic submucosal dissection for treatment of gastric subepithelial tumors (with video). Gastrointest Endosc. 2012; 75:276–286.
Article20. Tan Y, Tan L, Lu J, et al. Endoscopic resection of gastric gastrointestinal stromal tumors. Transl Gastroenterol Hepatol. 2017; 2:115.
Article21. Huberty V, Verset L, Deviere J. Endoscopic full-thickness resection of a gastric GI stromal tumor. VideoGIE. 2019; 4:120–122.
Article22. Guo JT, Zhang JJ, Wu YF, et al. Endoscopic full-thickness resection using an over-the-scope device: a prospective study. World J Gastroenterol. 2021; 27:725–736.
Article23. Schlag C, Wilhelm D, von Delius S, et al. EndoResect study: endoscopic full-thickness resection of gastric subepithelial tumors. Endoscopy. 2013; 45:4–11.
Article24. Meier B, Schmidt A, Glaser N, et al. Endoscopic full-thickness resection of gastric subepithelial tumors with the gFTRD-system: a prospective pilot study (RESET trial). Surg Endosc. 2020; 34:853–860.
Article25. Schmidt A, Bauder M, Riecken B, et al. Endoscopic full-thickness resection of gastric subepithelial tumors: a single-center series. Endoscopy. 2015; 47:154–158.
Article26. Inoue H, Ikeda H, Hosoya T, et al. Submucosal endoscopic tumor resection for subepithelial tumors in the esophagus and cardia. Endoscopy. 2012; 44:225–230.
Article27. Lv XH, Wang CH, Xie Y. Efficacy and safety of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors: a systematic review and meta-analysis. Surg Endosc. 2017; 31:49–63.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Endoscopic Full-Thickness Resection Combined with Laparoscopic Surgery
- Endoscopic Full-thickness Resection for Gastric Tumor
- Natural Orifice Transluminal Endoscopic Surgery and Upper Gastrointestinal Tract
- Omental Patching and Purse-String Endosuture Closure after Endoscopic Full-Thickness Resection in Patients with Gastric Gastrointestinal Stromal Tumors
- Endoscopic Full-Thickness Resection for Gastric Subepithelial Lesions Arising from the Muscularis Propria