Brain Tumor Res Treat.
2024 Apr;12(2):121-124. 10.14791/btrt.2024.0004.
Understanding the Brain-Heart Connection Through a Case of Angry Glioma Syndrome
- Affiliations
-
- 1St. Luke’s Medical Center, Institute for Neurosciences, Quezon City, Philippines
- KMID: 2555871
- DOI: http://doi.org/10.14791/btrt.2024.0004
Abstract
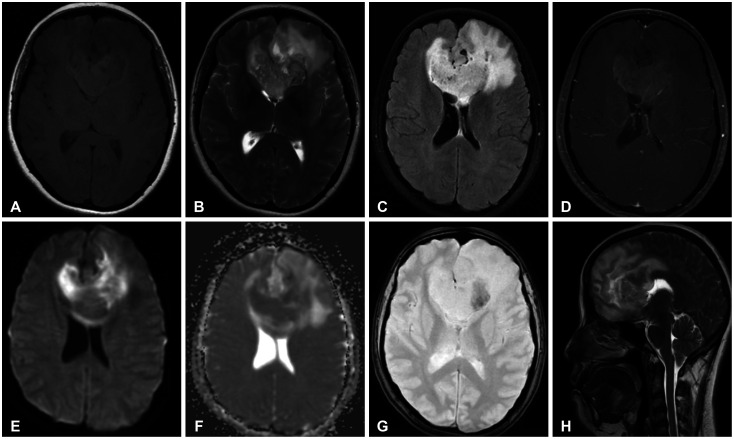
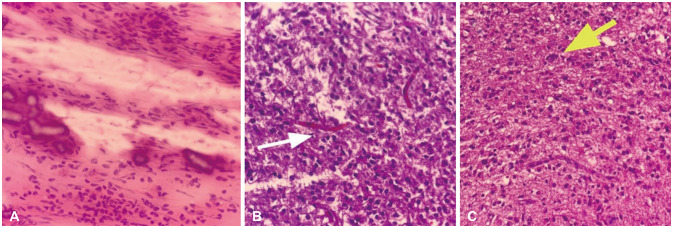
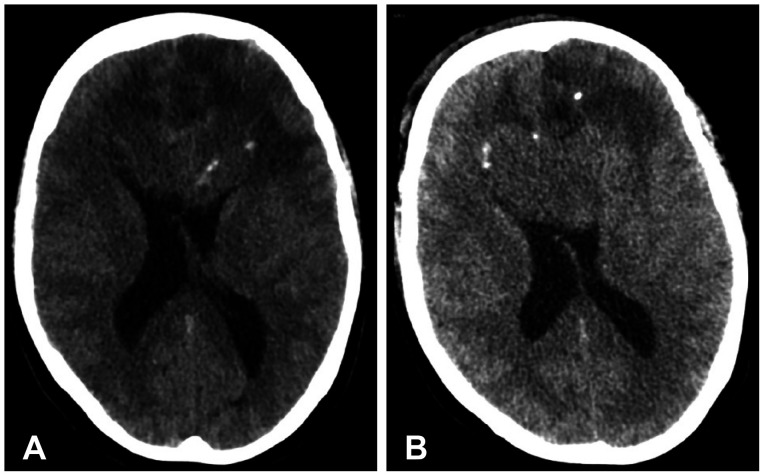
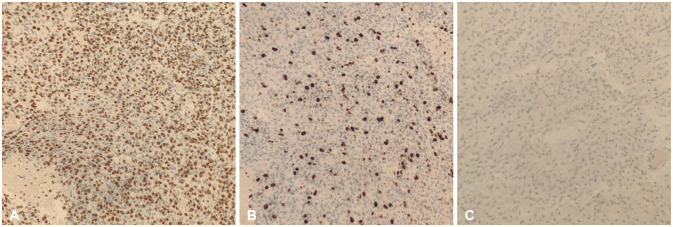
- We discuss a patient with a tumor on the anterior corpus callosum who underwent open biopsy eventually succumbing to cerebrogenic fatal arrhythmia following wounded glioma syndrome. A healthy 37-year-old female patient was admitted to our department due to a history of headache for 13 months. MRI revealed a suspicious glioma infiltrating the anterior corpus callosum. Neurologic examination only showed low cognitive assessment score (Montreal Cognitive Assessment score 20/30). ECG was normal sinus rhythm. Steroids and levetiracetam were administered prior to operation. Patient underwent right frontal craniotomy and biopsy of tumor with unremarkable events. During the first hospital day, patient had episodes of bradycardia followed by decrease in sensorium. Brain CT scan showed progression of edema without hemorrhage within the tumor bed. This was followed minutes later by two episodes of generalized tonic-clonic seizures and pulseless ventricular tachycardia. Cardiac resuscitation was done for 24 minutes but patient eventually expired. Location of the lesion and the epileptogenicity of the peritumoral cortex greatly contributed to the patient’s demise. Involvement of the frontomesial structures, particularly the insula and the cingulate cortex, and their connection to the central autonomic network, increased susceptibility to arrhythmias. Decreased seizure threshold worsened post-operative edema, further aggravating the dysregulation of the brain-heart-connection.
Keyword
Figure
Reference
-
1. Krajewski KL, Hernández-Durán S, Matschke J, Abboud T. Wounded glioma with fatal outcome: a report on biopsy of anaplastic glioma in two patients. Interdiscip Neurosurg. 2020; 19:100554.2. Hills KE, Kostarelos K, Wykes RC. Converging mechanisms of epileptogenesis and their insight in glioblastoma. Front Mol Neurosci. 2022; 15:903115. PMID: 35832394.3. Taggart P, Critchley H, Lambiase PD. Heart-brain interactions in cardiac arrhythmia. Heart. 2011; 97:698–708. PMID: 21367742.4. Koebbe CJ, Sherman JD, Warnick RE. Distant wounded glioma syndrome: report of two cases. Neurosurgery. 2001; 48:940–943. discussion 943-4. PMID: 11322457.5. Chrastina J, Novák Z, Ráha I, Slaná B, Jancálek R. [Distant wounded glioma syndrome following stereotactic biopsy--a case review]. Rozhl Chir. 2011; 90:148–151. Czech. PMID: 21634090.6. Buckingham SC, Robel S. Glutamate and tumor-associated epilepsy: glial cell dysfunction in the peritumoral environment. Neurochem Int. 2013; 63:696–701. PMID: 23385090.7. Robert SM, Sontheimer H. Glutamate transporters in the biology of malignant gliomas. Cell Mol Life Sci. 2014; 71:1839–1854. PMID: 24281762.8. Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011; 8:665–670. PMID: 21706013.9. Uddin LQ. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci. 2015; 16:55–61. PMID: 25406711.10. Pchejetski D, Kenbaz M, Alshaker H, Jesudason K. Bradycardia and syncope as sole manifestations of a cranial lesion: a case report. J Med Case Rep. 2020; 14:24. PMID: 32000857.11. Benarroch EE. Central autonomic network: functional organization and clinical correlations. Armonk: Futura Publishing Company;1997.12. Palma JA, Benarroch EE. Neural control of the heart: recent concepts and clinical correlations. Neurology. 2014; 83:261–271. PMID: 24928126.13. Witt CM, Bolona L, Kinney MO, Moir C, Ackerman MJ, Kapa S, et al. Denervation of the extrinsic cardiac sympathetic nervous system as a treatment modality for arrhythmia. Europace. 2017; 19:1075–1083. PMID: 28340164.