Diabetes Metab J.
2024 May;48(3):473-481. 10.4093/dmj.2023.0370.
Switching from Conventional Fibrates to Pemafibrate Has Beneficial Effects on the Renal Function of Diabetic Subjects with Chronic Kidney Disease
- Affiliations
-
- 1Department of Rheumatology, Endocrinology and Nephrology, Faculty of Medicine and Graduate School of Medicine, Hokkaido University, Sapporo, Japan
- 2Division of Diabetes and Endocrinology, Department of Medicine, NTT Sapporo Medical Center, Sapporo, Japan
- 3Caress Sapporo Hokko Memorial Clinic, Sapporo, Japan
- KMID: 2555778
- DOI: http://doi.org/10.4093/dmj.2023.0370
Abstract
- Background
Fibrates have renal toxicity limiting their use in subjects with chronic kidney disease (CKD). However, pemafibrate has fewer toxic effects on renal function. In the present analysis, we evaluated the effects of pemafibrate on the renal function of diabetic subjects with or without CKD in a real-world clinical setting.
Methods
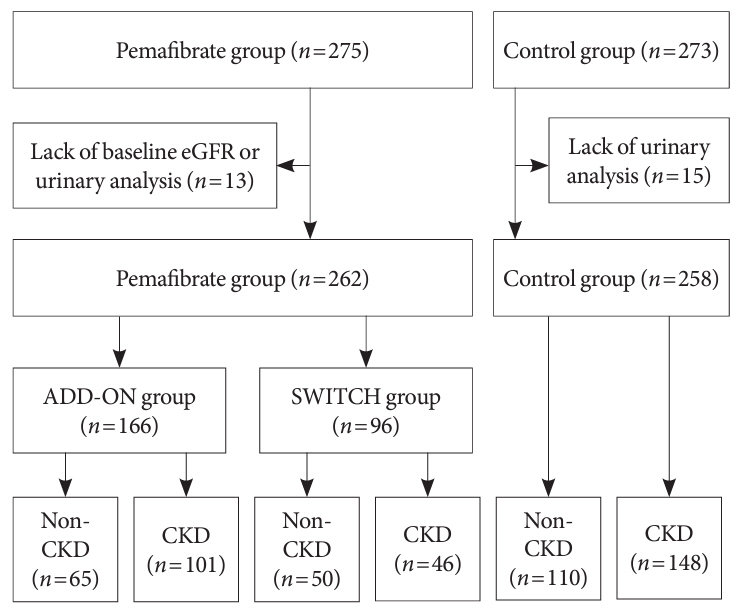
We performed a sub-analysis of data collected during a multi-center, prospective, observational study of the effects of pemafibrate on lipid metabolism in subjects with type 2 diabetes mellitus complicated by hypertriglyceridemia (the PARM-T2D study). The participants were allocated to add pemafibrate to their existing regimen (ADD-ON), switch from their existing fibrate to pemafibrate (SWITCH), or continue conventional therapy (CTRL). The changes in estimated glomerular filtration rate (eGFR) over 52 weeks were compared among these groups as well as among subgroups created according to CKD status.
Results
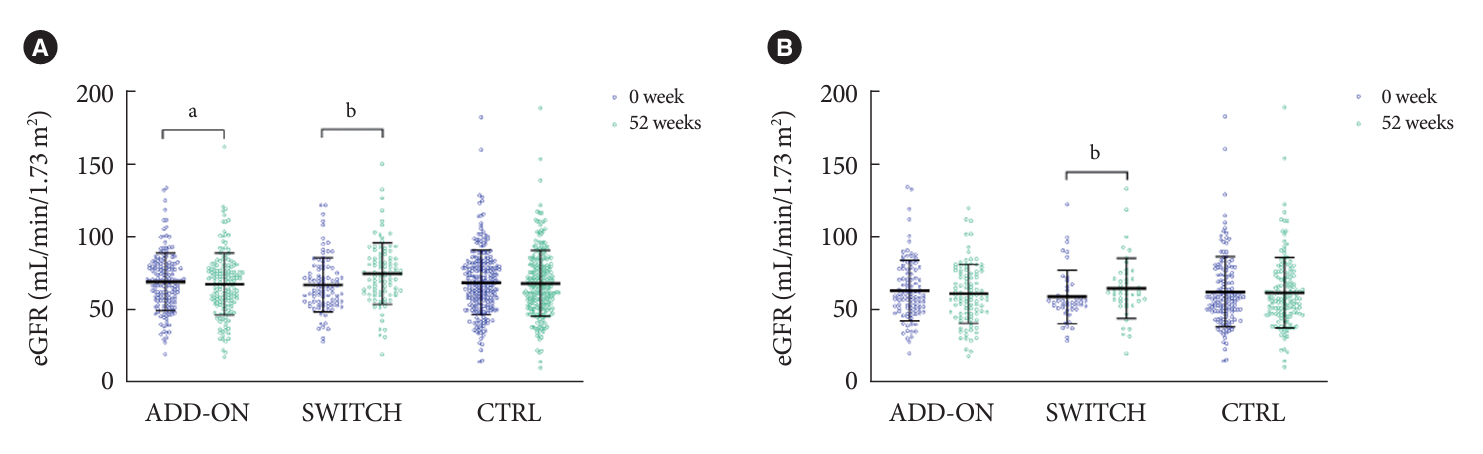
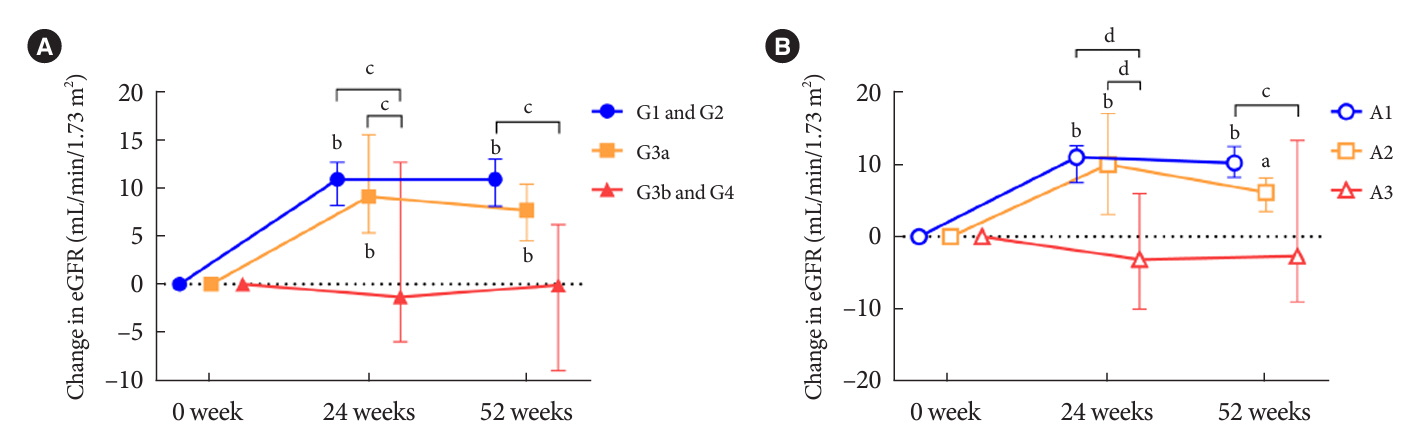
Data for 520 participants (ADD-ON, n=166; SWITCH, n=96; CTRL, n=258) were analyzed. Of them, 56.7% had CKD. The eGFR increased only in the SWITCH group, and this trend was also present in the CKD subgroup (P<0.001). On the other hand, eGFR was not affected by switching in participants with severe renal dysfunction (G3b or G4) and/or macroalbuminuria. Multivariate analysis showed that being older and a switch from fenofibrate were associated with elevation in eGFR (both P<0.05).
Conclusion
A switch to pemafibrate may be associated with an elevation in eGFR, but to a lesser extent in patients with poor renal function.
Figure
Reference
-
1. Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR; UKPDS Study Group. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes. 2006; 55:1832–9.2. Kung K, Chow KM, Hui EM, Leung M, Leung SY, Szeto CC, et al. Prevalence of complications among Chinese diabetic patients in urban primary care clinics: a cross-sectional study. BMC Fam Pract. 2014; 15:8.
Article3. Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017; 12:2032–45.4. Davis TM, Ting R, Best JD, Donoghoe MW, Drury PL, Sullivan DR, et al. Effects of fenofibrate on renal function in patients with type 2 diabetes mellitus: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) Study. Diabetologia. 2011; 54:280–90.
Article5. Ishibashi S, Arai H, Yokote K, Araki E, Suganami H, Yamashita S, et al. Efficacy and safety of pemafibrate (K-877), a selective peroxisome proliferator-activated receptor α modulator, in patients with dyslipidemia: results from a 24-week, randomized, double blind, active-controlled, phase 3 trial. J Clin Lipidol. 2018; 12:173–84.6. Das Pradhan A, Glynn RJ, Fruchart JC, MacFadyen JG, Zaharris ES, Everett BM, et al. Triglyceride lowering with pemafibrate to reduce cardiovascular risk. N Engl J Med. 2022; 387:1923–34.
Article7. Kito K, Nomoto H, Sakuma I, Nakamura A, Cho KY, Kameda H, et al. Effects of pemafibrate on lipid metabolism in patients with type 2 diabetes and hypertriglyceridemia: a multi-center prospective observational study, the PARM-T2D study. Diabetes Res Clin Pract. 2022; 192:110091.
Article8. de Boer IH, Khunti K, Sadusky T, Tuttle KR, Neumiller JJ, Rhee CM, et al. Diabetes management in chronic kidney disease: a consensus report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Diabetes Care. 2022; 45:3075–90.
Article9. Ansquer JC, Foucher C, Rattier S, Taskinen MR, Steiner G; DAIS Investigators. Fenofibrate reduces progression to microalbuminuria over 3 years in a placebo-controlled study in type 2 diabetes: results from the Diabetes Atherosclerosis Intervention Study (DAIS). Am J Kidney Dis. 2005; 45:485–93.
Article10. Hadjivasilis A, Kouis P, Kousios A, Panayiotou A. The effect of fibrates on kidney function and chronic kidney disease progression: a systematic review and meta-analysis of randomised studies. J Clin Med. 2022; 11:768.
Article11. Bonds DE, Craven TE, Buse J, Crouse JR, Cuddihy R, Elam M, et al. Fenofibrate-associated changes in renal function and relationship to clinical outcomes among individuals with type 2 diabetes: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) experience. Diabetologia. 2012; 55:1641–50.
Article12. Hottelart C, El Esper N, Rose F, Achard JM, Fournier A. Fenofibrate increases creatininemia by increasing metabolic production of creatinine. Nephron. 2002; 92:536–41.
Article13. Chen YJ, Quilley J. Fenofibrate treatment of diabetic rats reduces nitrosative stress, renal cyclooxygenase-2 expression, and enhanced renal prostaglandin release. J Pharmacol Exp Ther. 2008; 324:658–63.
Article14. Attridge RL, Linn WD, Ryan L, Koeller J, Frei CR. Evaluation of the incidence and risk factors for development of fenofibrate-associated nephrotoxicity. J Clin Lipidol. 2012; 6:19–26.
Article15. Mandard S, Muller M, Kersten S. Peroxisome proliferator-activated receptor alpha target genes. Cell Mol Life Sci. 2004; 61:393–416.16. Schiffrin EL. Peroxisome proliferator-activated receptors and cardiovascular remodeling. Am J Physiol Heart Circ Physiol. 2005; 288:H1037–43.
Article17. Stulc T, Kasalova Z, Krejci H, Dolezalova R, Ceska R. Effect of rosiglitazone on homocysteine and creatinine levels in patients with type 2 diabetes. Atherosclerosis. 2005; 183:367–8.
Article18. Maki T, Maeda Y, Sonoda N, Makimura H, Kimura S, Maeno S, et al. Renoprotective effect of a novel selective PPARα modulator K-877 in db/db mice: a role of diacylglycerol-protein kinase C-NAD(P)H oxidase pathway. Metabolism. 2017; 71:33–45.
Article19. Zhang J, Ji X, Dong Z, Lu J, Zhao Y, Li R, et al. Impact of fenofibrate therapy on serum uric acid concentrations: a review and meta-analysis. Endocr J. 2021; 68:829–37.
Article20. Wang JC, Bennett M. Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ Res. 2012; 111:245–59.21. Scuteri A, Najjar SS, Morrell CH, Lakatta EG; Cardiovascular Health Study. The metabolic syndrome in older individuals: prevalence and prediction of cardiovascular events: the Cardiovascular Health Study. Diabetes Care. 2005; 28:882–7.22. Gigante A, Proietti M, Petrillo E, Mannucci PM, Nobili A, Muscaritoli M, et al. Renal function, cardiovascular diseases, appropriateness of drug prescription and outcomes in hospitalized older patients. Drugs Aging. 2021; 38:1097–105.
Article23. Hayashi C. What is the role of statistics in medical science?: a critical essay. Gan To Kagaku Ryoho. 1992; 19:143–59.24. Hounslow N, Mair S, Suganami H, Nakamura M. Pemafibrate has high bioavailability and is principally excreted via the liver. Atheroscler Suppl. 2018; 32:157.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Switching from Fenofibrate to Pemafibrate for Asymptomatic Primary Biliary Cholangitis
- Diagnosis and test for diabetic kidney disease
- Glycemic Control in Diabetic Patients with Diabetic Nephropathy
- The Epidemiology of Diabetic Nephropathy
- Use of dapagliflozin in patients with advanced diabetic kidney disease