Diabetes Metab J.
2024 May;48(3):418-428. 10.4093/dmj.2023.0030.
Safety and Effectiveness of Dulaglutide in the Treatment of Type 2 Diabetes Mellitus: A Korean Real-World Post-Marketing Study
- Affiliations
-
- 1Lilly Korea Ltd., Seoul, Korea
- 2Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 3Department of Internal Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 4Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 5Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 6Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2555773
- DOI: http://doi.org/10.4093/dmj.2023.0030
Abstract
- Background
To investigate the real-world safety and effectiveness of dulaglutide in Korean adults with type 2 diabetes mellitus (T2DM).
Methods
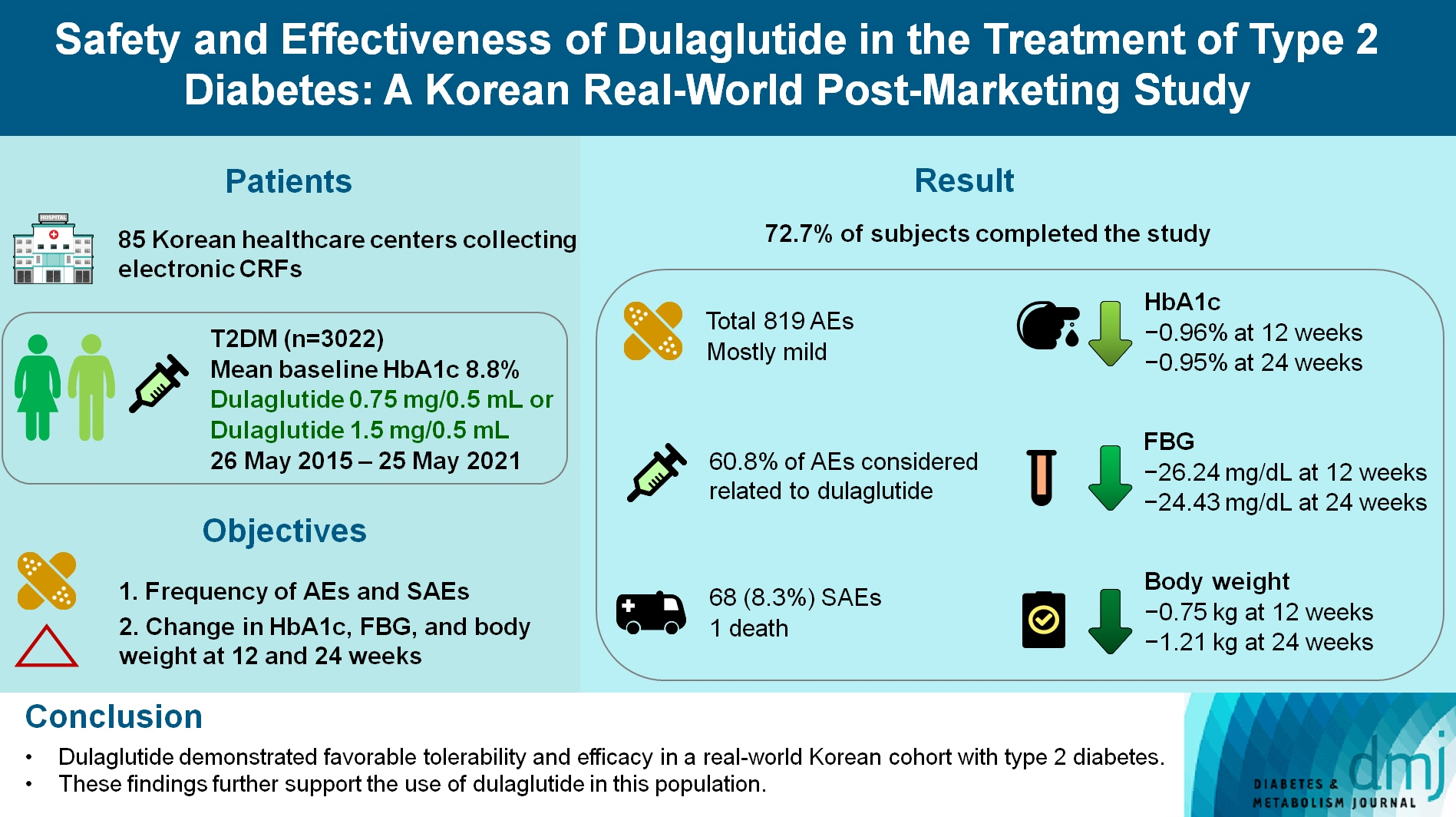
This was a real-world, prospective, non-interventional post-marketing safety study conducted from May 26, 2015 to May 25, 2021 at 85 Korean healthcare centers using electronic case data. Data on patients using dulaglutide 0.75 mg/0.5 mL or the dulaglutide 1.5 mg/0.5 mL single-use pens were collected and pooled. The primary objective was to report the frequency and proportion of adverse and serious adverse events that occurred. The secondary objective was to monitor the effectiveness of dulaglutide at 12 and 24 weeks by evaluating changes in glycosylated hemoglobin (HbA1c ), fasting plasma glucose, and body weight.
Results
Data were collected from 3,067 subjects, and 3,022 subjects who received ≥1 dose (of any strength) of dulaglutide were included in the safety analysis set (53% female, mean age 56 years; diabetes duration 11.2 years, mean HbA1c 8.8%). The number of adverse events reported was 819; of these, 68 (8.3%) were serious adverse events. One death was reported. Adverse events were mostly mild in severity; 60.81% of adverse events were considered related to dulaglutide. This study was completed by 72.73% (2,198/3,022) of subjects. At 12/24 weeks there were significant (P<0.0001) reductions from baseline in least-squares mean HbA1c (0.96%/0.95%), fasting blood glucose (26.24/24.43 mg/dL), and body weight (0.75/1.21 kg).
Conclusion
Dulaglutide was generally well tolerated and effective in real-world Korean individuals with T2DM. The results from this study contribute to the body of evidence for dulaglutide use in this population.
Keyword
Figure
Reference
-
1. Korean Diabetes Association. Diabetes facts sheet in Korea. Seoul: KDA;2022.2. Andreasen CR, Andersen A, Knop FK, Vilsboll T. Understanding the place for GLP-1RA therapy: translating guidelines for treatment of type 2 diabetes into everyday clinical practice and patient selection. Diabetes Obes Metab. 2021; 23 Suppl 3:40–52.3. Zhang L, Zhang M, Zhang Y, Tong N. Efficacy and safety of dulaglutide in patients with type 2 diabetes: a meta-analysis and systematic review. Sci Rep. 2016; 6:18904.
Article4. Pace E, Tingen J. Dulaglutide (trulicity) for type 2 diabetes mellitus. Am Fam Physician. 2017; 96:540–2.5. Glaesner W, Vick AM, Millican R, Ellis B, Tschang SH, Tian Y, et al. Engineering and characterization of the long-acting glucagon-like peptide-1 analogue LY2189265, an Fc fusion protein. Diabetes Metab Res Rev. 2010; 26:287–96.
Article6. Sheahan KH, Wahlberg EA, Gilbert MP. An overview of GLP-1 agonists and recent cardiovascular outcomes trials. Postgrad Med J. 2020; 96:156–61.
Article7. Oh S, Chon S, Ahn KJ, Jeong IK, Kim BJ, Kang JG. The role of glucagon-like peptide-1 receptor agonists in type 2 diabetes: understanding how data can inform clinical practice in Korea. Diabetes Metab J. 2015; 39:177–87.
Article8. Kang YM, Cho YK, Lee J, Lee SE, Lee WJ, Park JY, et al. Asian subpopulations may exhibit greater cardiovascular benefit from long-acting glucagon-like peptide 1 receptor agonists: a meta-analysis of cardiovascular outcome trials. Diabetes Metab J. 2019; 43:410–21.
Article9. Onakpoya I. Rare adverse events in clinical trials: understanding the rule of three. Available from: https://blogs.bmj.com/bmjebmspotlight/2017/11/14/rare-adverse-events-clinical-trials-understanding-rule-three/ (cited 2023 Aug 22).
Article10. European Commission. A guideline on summary of product characteristics 2009. Available from: https://health.ec.europa.eu/system/files/2016-11/smpc_guideline_rev2_en_0.pdf (cited 2023 Aug 22).11. Onishi Y, Oura T, Matsui A, Matsuura J, Iwamoto N. Analysis of efficacy and safety of dulaglutide 0.75 mg stratified by sex in patients with type 2 diabetes in 2 randomized, controlled phase 3 studies in Japan. Endocr J. 2017; 64:553–60.
Article12. Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomized placebo-controlled trial. Lancet. 2019; 394:121–30.13. Yu M, Yuan GY, Zhang B, Wu HY, Lv XF. Efficacy and safety of dulaglutide by baseline HbA1c in Chinese patients with type 2 diabetes: a post hoc analysis. Diabetes Ther. 2020; 11:1147–59.
Article14. Yoon JH, Hong AR, Choi W, Park JY, Kim HK, Kang HC. Real-world efficacy and safety of dulaglutide in Korean patients with type 2 diabetes mellitus: a retrospective study in a tertiary referral center. Chonnam Med J. 2021; 57:211–8.
Article15. Lee J, Cho YK, Kim HS, Jung CH, Park JY, Lee WJ. Dulaglutide as an add-on to insulin in type 2 diabetes: clinical efficacy and parameters affecting the response in real-world practice. Diabetes Metab Syndr Obes. 2019; 12:2745–53.16. Gallwitz B, Dagogo-Jack S, Thieu V, Garcia-Perez LE, Pavo I, Yu M, et al. Effect of once-weekly dulaglutide on glycated haemoglobin (HbA1c) and fasting blood glucose in patient subpopulations by gender, duration of diabetes and baseline HbA1c. Diabetes Obes Metab. 2018; 20:409–18.17. Yu Y, Chen J, Li D, Wang L, Wang W, Liu H. Systematic analysis of adverse event reports for sex differences in adverse drug events. Sci Rep. 2016; 6:24955.
Article18. Bechman K, Clarke BD, Rutherford AI, Yates M, Nikiphorou E, Molokhia M, et al. Polypharmacy is associated with treatment response and serious adverse events: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Rheumatology (Oxford). 2019; 58:1767–76.
Article19. Modesto AC, Silveira EA, de Carvalho Santos AS, dos Santos Rodrigues AP, Lima DM, Provin MP, et al. Prevalence of adverse drug events in severely obese adults and associated factors: clinical trial baseline results. Sci Pharm. 2020; 88:41.
Article20. Woo SD, Yoon J, Doo GE, Park Y, Lee Y, Lee SH, et al. Common causes and characteristics of adverse drug reactions in older adults: a retrospective study. BMC Pharmacol Toxicol. 2020; 21:87.
Article21. Lavan AH, Gallagher P. Predicting risk of adverse drug reactions in older adults. Ther Adv Drug Saf. 2016; 7:11–22.
Article22. Gomes IV, Muniz CR, Vieira RS, Reis RL, Carmo RF, Silva DT. Risk factors for adverse drug events in hospitalized patients: an overview of systematic reviews. Rev Bras Farm Hosp Serv Saude. 2022; 13:0738.
Article23. Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet. 2019; 394:131–8.24. Kwan AY, Gerstein HC, Basile J, Xavier D, Maldonado JM, Raha S, et al. HbA1c reduction in dulaglutide-treated patients irrespective of duration of diabetes, microvascular disease, and BMI: a post hoc analysis from the REWIND trial. Diabetes Care. 2022; 45:547–54.
Article25. Mody R, Yu M, Grabner M, Boye K, Teng CC, Kwan AY. Dulaglutide shows sustained reduction in glycosylated hemoglobin values: 2-year US real-world study results. Clin Ther. 2020; 42:2184–95.
Article26. Yoo JH, Cho YK, Lee J, Kim HS, Kang YM, Jung CH, et al. Clinical efficacy and parameters affecting the response to dulaglutide treatment in patients with type 2 diabetes: a retrospective, real-world data study. Diabetes Ther. 2019; 10:1453–63.
Article27. Kim SS, Kim IJ, Kim YK, Yoon KH, Son HY, Park SW, et al. Duration of diabetes and effectiveness of insulin in the management of insulin-naive Korean patients uncontrolled on oral antidiabetic drugs: a sub-analysis of the MOdaliTy of Insulin treatment eValuation (MOTIV) registry results. Acta Diabetol. 2014; 51:655–61.
Article28. Trujillo JM, Nuffer W, Smith BA. GLP-1 receptor agonists: an updated review of head-to-head clinical studies. Ther Adv Endocrinol Metab. 2021; 12:2042018821997320.
Article29. Bonora E, Frias JP, Tinahones FJ, Van J, Malik RE, Yu Z, et al. Effect of dulaglutide 3.0 and 4.5 mg on weight in patients with type 2 diabetes: exploratory analyses of AWARD-11. Diabetes Obes Metab. 2021; 23:2242–50.30. Hong YH, Chung IH, Han K, Chung S; Taskforce Team of the Obesity Fact Sheet of the Korean Society for the Study of Obesity. Prevalence of type 2 diabetes mellitus among Korean children, adolescents, and adults younger than 30 years: changes from 2002 to 2016. Diabetes Metab J. 2022; 46:297–306.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Real-World Efficacy and Safety of Dulaglutide in Korean Patients with Type 2 Diabetes Mellitus: A Retrospective Study in a Tertiary Referral Center
- Dulaglutide as an Effective Replacement for Prandial Insulin in Kidney Transplant Recipients with Type 2 Diabetes Mellitus: A Retrospective Review
- Current Status of Post-marketing Safety Management in United States, Europe and Japan: Risk Management Plan
- Safety of COVID-19 Vaccines among Patients with Type 2 Diabetes Mellitus: Real-World Data Analysis (Diabetes Metab J 2023;47:356-65)
- Safety and efficacy of fimasartan with essential hypertension patients in real world clinical practice: data from a post marketing surveillance in Korea