Diabetes Metab J.
2024 May;48(3):390-404. 10.4093/dmj.2023.0109.
Supplementation of Clostridium butyricum Alleviates Vascular Inflammation in Diabetic Mice
- Affiliations
-
- 1Department of Ultrasound Diagnostics, Tangdu Hospital, Air Force Medical University, Xi’an, China
- 2Department of Aerospace Medicine, Air Force Medical University, Xi’an, China
- 3Department of Biochemistry and Molecular Biology, Air Force Medical University, Xi’an, China
- 4Department of Ultrasound Diagnostics, The PLA Rocket Force Characteristic Medical Center, Beijing, China
- KMID: 2555771
- DOI: http://doi.org/10.4093/dmj.2023.0109
Abstract
- Background
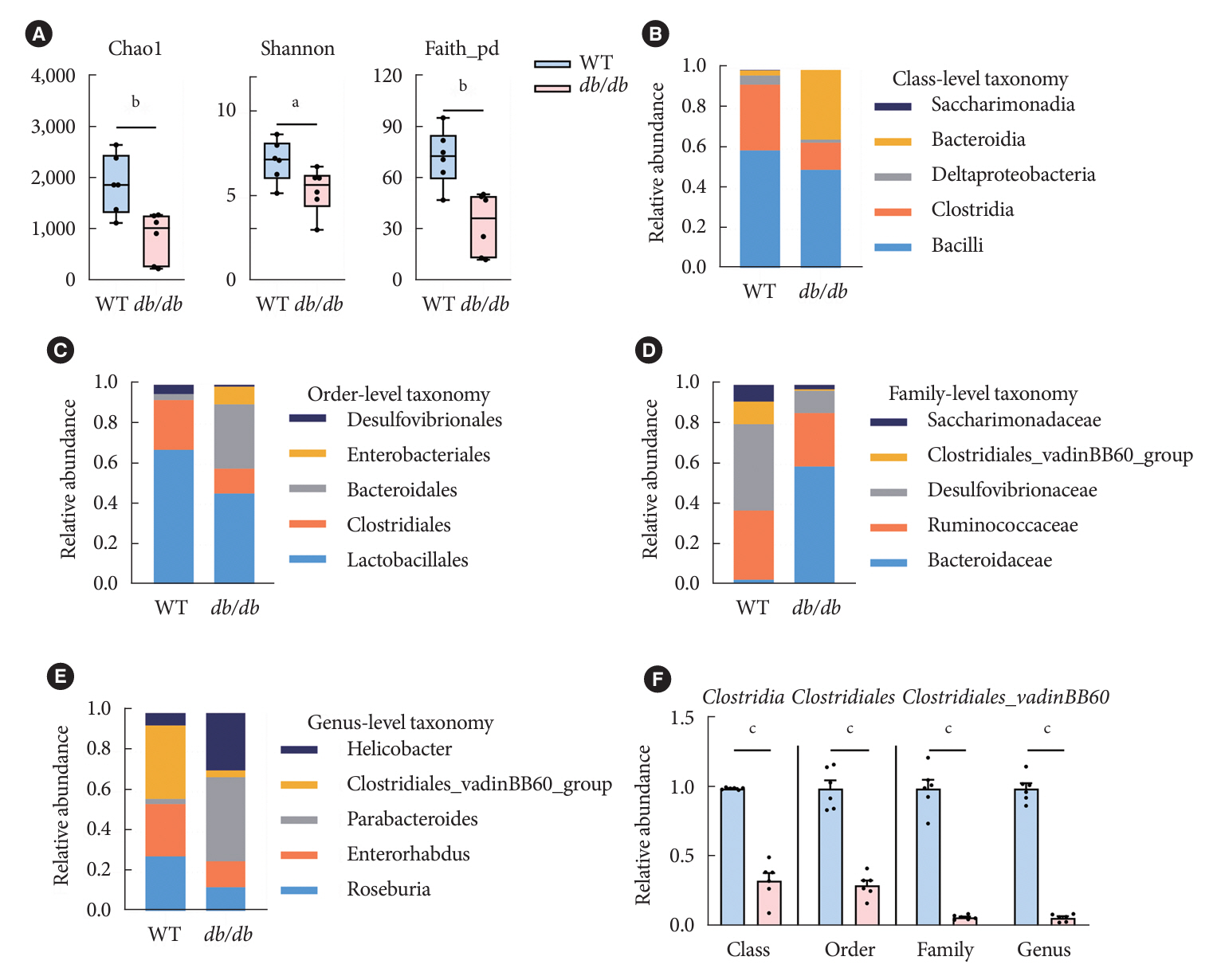
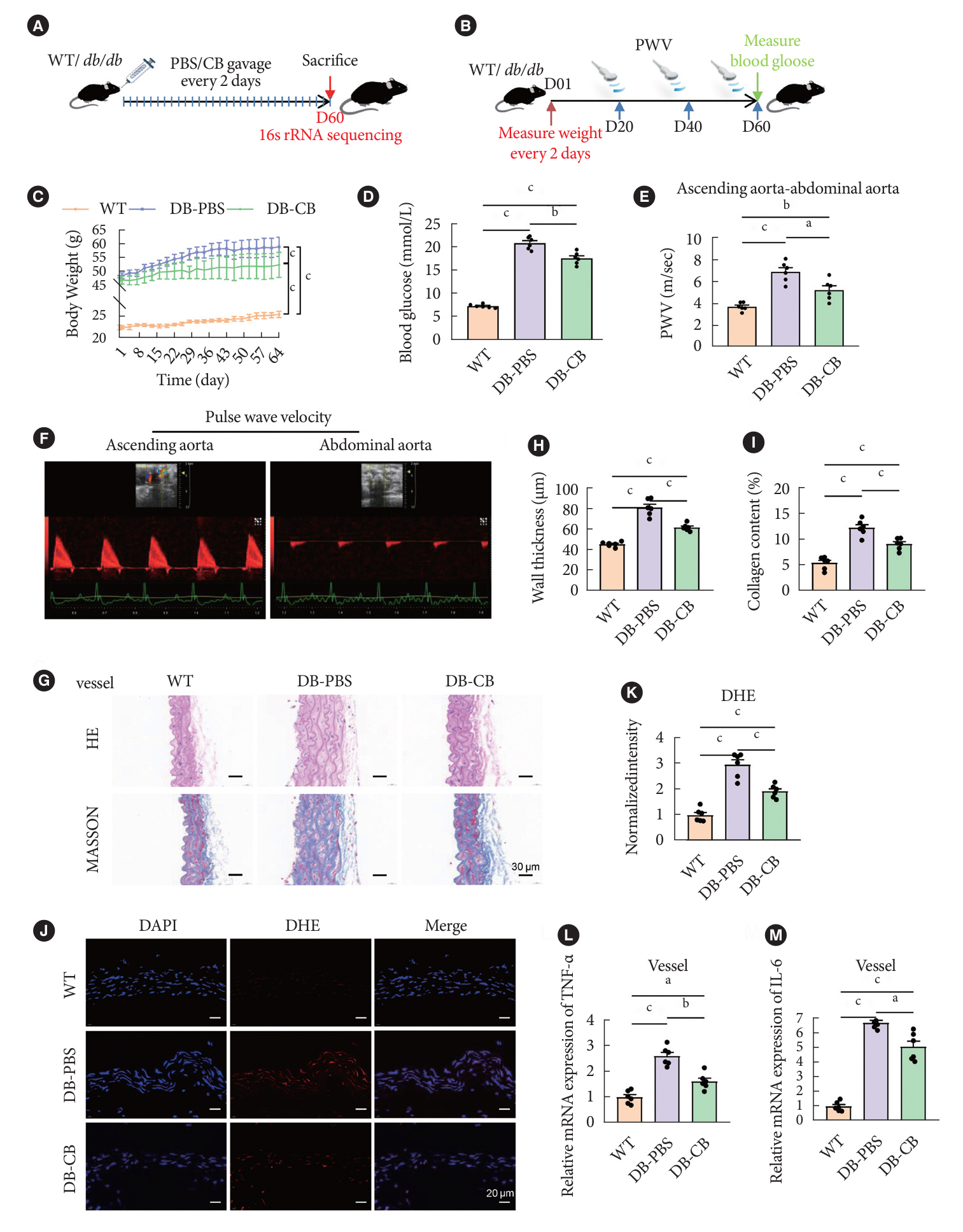
Gut microbiota is closely related to the occurrence and development of diabetes and affects the prognosis of diabetic complications, and the underlying mechanisms are only partially understood. We aimed to explore the possible link between the gut microbiota and vascular inflammation of diabetic mice.
Methods
The db/db diabetic and wild-type (WT) mice were used in this study. We profiled gut microbiota and examined the and vascular function in both db/db group and WT group. Gut microbiota was analyzed by 16s rRNA sequencing. Vascular function was examined by ultrasonographic hemodynamics and histological staining. Clostridium butyricum (CB) was orally administered to diabetic mice by intragastric gavage every 2 days for 2 consecutive months. Reactive oxygen species (ROS) and expression of nuclear factor erythroid-derived 2-related factor 2 (Nrf2) and heme oxygenase-1 (HO-1) were detected by fluorescence microscopy. The mRNA expression of inflammatory cytokines was tested by quantitative polymerase chain reaction.
Results
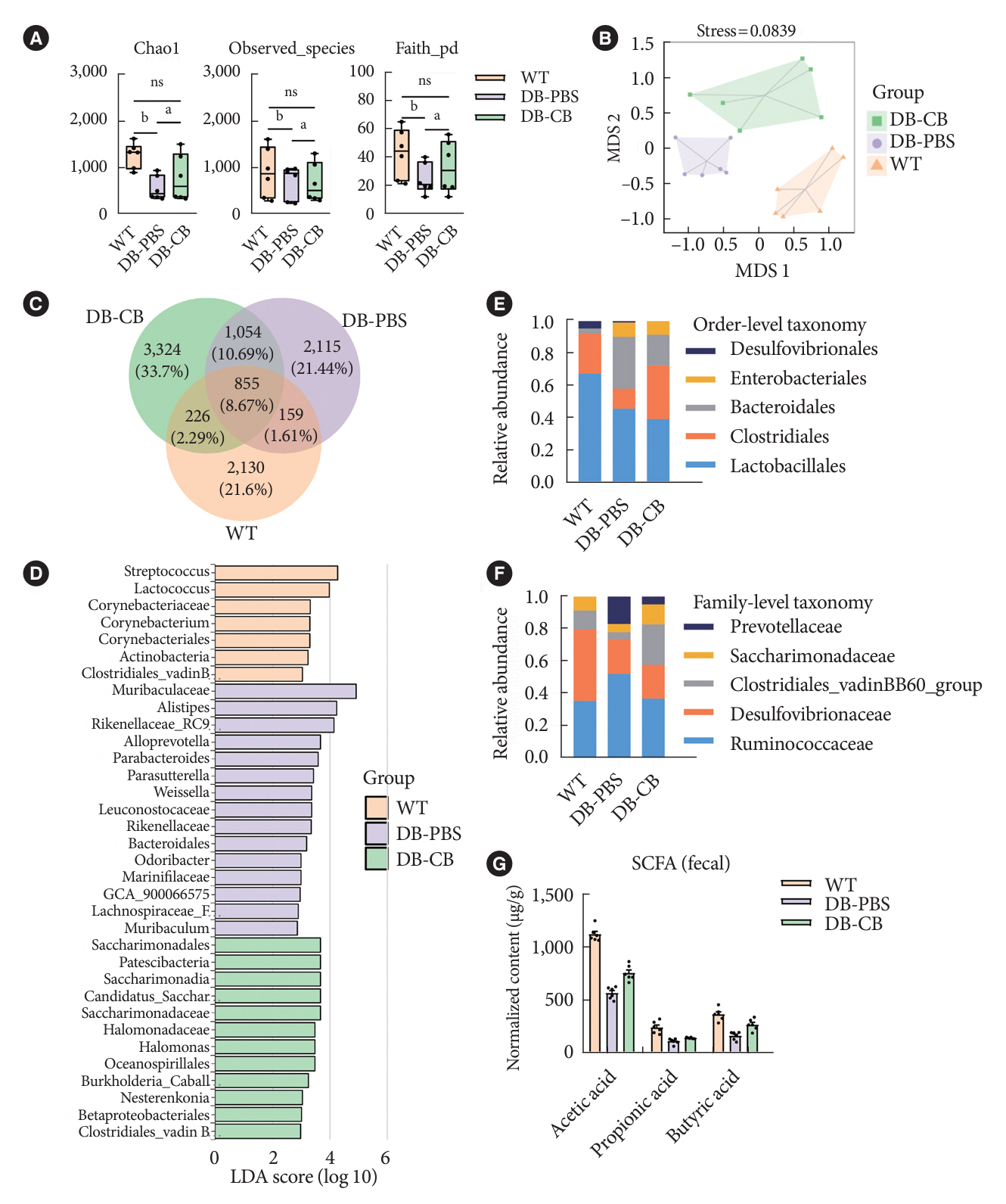
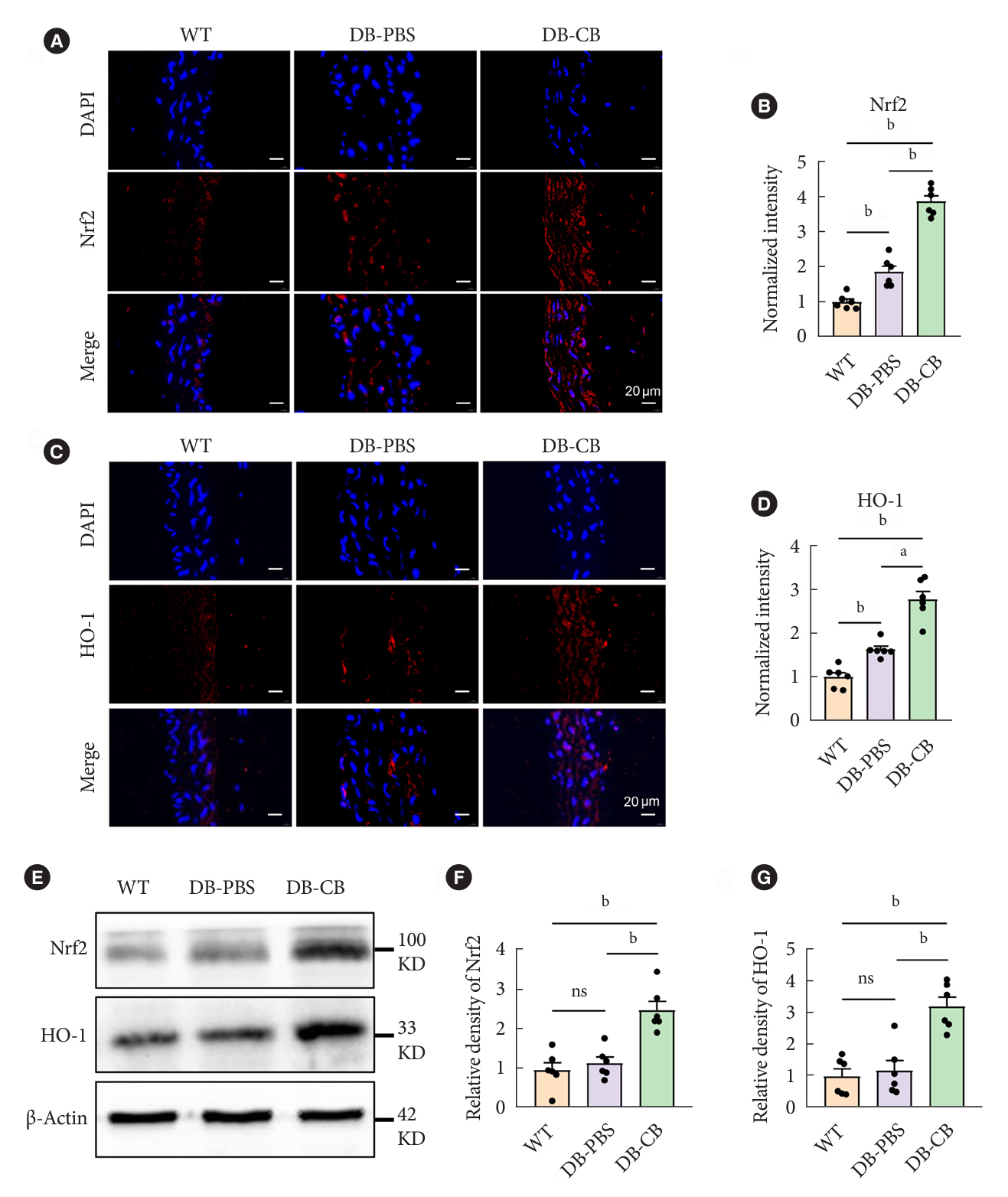
Compared with WT mice, CB abundance was significantly decreased in the gut of db/db mice, together with compromised vascular function and activated inflammation in the arterial tissue. Meanwhile, ROS in the vascular tissue of db/db mice was also significantly increased. Oral administration of CB restored the protective microbiota, and protected the vascular function in the db/db mice via activating the Nrf2/HO-1 pathway.
Conclusion
This study identified the potential link between decreased CB abundance in gut microbiota and vascular inflammation in diabetes. Therapeutic delivery of CB by gut transplantation alleviates the vascular lesions of diabetes mellitus by activating the Nrf2/HO-1 pathway.
Figure
Reference
-
1. Pearson-Stuttard J, Cheng YJ, Bennett J, Vamos EP, Zhou B, Valabhji J, et al. Trends in leading causes of hospitalisation of adults with diabetes in England from 2003 to 2018: an epidemiological analysis of linked primary care records. Lancet Diabetes Endocrinol. 2022; 10:46–57.
Article2. Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol. 2014; 10:293–302.
Article3. Paneni F, Costantino S, Cosentino F. Insulin resistance, diabetes, and cardiovascular risk. Curr Atheroscler Rep. 2014; 16:419.
Article4. Hill MA, Yang Y, Zhang L, Sun Z, Jia G, Parrish AR, et al. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism. 2021; 119:154766.
Article5. James DE, Stockli J, Birnbaum MJ. The aetiology and molecular landscape of insulin resistance. Nat Rev Mol Cell Biol. 2021; 22:751–71.
Article6. Tai N, Wong FS, Wen L. The role of gut microbiota in the development of type 1, type 2 diabetes mellitus and obesity. Rev Endocr Metab Disord. 2015; 16:55–65.
Article7. Ruan W, Engevik MA, Spinler JK, Versalovic J. Healthy human gastrointestinal microbiome: composition and function after a decade of exploration. Dig Dis Sci. 2020; 65:695–705.
Article8. Jia G, DeMarco VG, Sowers JR. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol. 2016; 12:144–53.
Article9. Defronzo RA. Banting lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009; 58:773–95.10. Rodriguez ML, Perez S, Mena-Molla S, Desco MC, Ortega AL. Oxidative stress and microvascular alterations in diabetic retinopathy: future therapies. Oxid Med Cell Longev. 2019; 2019:4940825.11. Li F, Wang M, Wang J, Li R, Zhang Y. Alterations to the gut microbiota and their correlation with inflammatory factors in chronic kidney disease. Front Cell Infect Microbiol. 2019; 9:206.
Article12. Li X, Wang Y, Zhou J, Wang Z, Wang Y, Zheng J, et al. Mixed nuts with high nutrient density improve insulin resistance in mice by gut microbiota remodeling. Food Funct. 2022; 13:9904–17.
Article13. Hao J, Zhang Y, Wu T, Liu R, Sui W, Zhu J, et al. The antidiabetic effects of Bifidobacterium longum subsp. longum BL21 through regulating gut microbiota structure in type 2 diabetic mice. Food Funct. 2022; 13:9947–58.14. Zhao D, Zhu H, Gao F, Qian Z, Mao W, Yin Y, et al. Antidiabetic effects of selenium-enriched Bifidobacterium longum DD98 in type 2 diabetes model of mice. Food Funct. 2020; 11:6528–41.15. Butlin M, Tan I, Spronck B, Avolio AP. Measuring arterial stiffness in animal experimental studies. Arterioscler Thromb Vasc Biol. 2020; 40:1068–77.
Article16. Wang C, Xing C, Li Z, Liu Y, Li Q, Wang Y, et al. Bioinspired therapeutic platform based on extracellular vesicles for prevention of arterial wall remodeling in hypertension. Bioact Mater. 2021; 8:494–504.
Article17. Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE, et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015; 519:92–6.
Article18. Cho I, Yamanishi S, Cox L, Methe BA, Zavadil J, Li K, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012; 488:621–6.
Article19. Maddahi A, Edvinsson L. Cerebral ischemia induces microvascular pro-inflammatory cytokine expression via the MEK/ERK pathway. J Neuroinflammation. 2010; 7:14.
Article20. Hervera A, De Virgiliis F, Palmisano I, Zhou L, Tantardini E, Kong G, et al. Reactive oxygen species regulate axonal regeneration through the release of exosomal NADPH oxidase 2 complexes into injured axons. Nat Cell Biol. 2018; 20:307–19.
Article21. Zhou J, Zhang L, Zheng B, Zhang L, Qin Y, Zhang X, et al. Salvia miltiorrhiza bunge exerts anti-oxidative effects through inhibiting KLF10 expression in vascular smooth muscle cells exposed to high glucose. J Ethnopharmacol. 2020; 262:113208.
Article22. Hamzah N, Safuan S, Wan Ishak WR. Potential effect of polyphenolic-rich fractions of corn silk on protecting endothelial cells against high glucose damage using in vitro and in vivo approaches. Molecules. 2021; 26:3665.
Article23. Xu YH, Gao CL, Guo HL, Zhang WQ, Huang W, Tang SS, et al. Sodium butyrate supplementation ameliorates diabetic inflammation in db/db mice. J Endocrinol. 2018; 238:231–44.
Article24. Yang T, Yang H, Heng C, Wang H, Chen S, Hu Y, et al. Amelioration of non-alcoholic fatty liver disease by sodium butyrate is linked to the modulation of intestinal tight junctions in db/db mice. Food Funct. 2020; 11:10675–89.
Article25. Mthiyane FT, Dludla PV, Ziqubu K, Mthembu SX, Muvhulawa N, Hlengwa N, et al. A review on the antidiabetic properties of Moringa oleifera extracts: focusing on oxidative stress and inflammation as main therapeutic targets. Front Pharmacol. 2022; 13:940572.
Article26. Wang H, Lu Y, Yan Y, Tian S, Zheng D, Leng D, et al. Promising treatment for type 2 diabetes: fecal microbiota transplantation reverses insulin resistance and impaired islets. Front Cell Infect Microbiol. 2020; 9:455.
Article27. Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019; 25:1822–32.
Article28. Beckman JA, Paneni F, Cosentino F, Creager MA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part II. Eur Heart J. 2013; 34:2444–52.
Article29. Machate DJ, Figueiredo PS, Marcelino G, Guimaraes RC, Hiane PA, Bogo D, et al. Fatty acid diets: regulation of gut microbiota composition and obesity and its related metabolic dysbiosis. Int J Mol Sci. 2020; 21:4093.
Article30. Xu J, Lian F, Zhao L, Zhao Y, Chen X, Zhang X, et al. Structural modulation of gut microbiota during alleviation of type 2 diabetes with a Chinese herbal formula. ISME J. 2015; 9:552–62.
Article31. Avagliano C, De Caro C, Cuozzo M, Liguori FM, La Rana G, Micheli L, et al. Phaseolus vulgaris extract ameliorates high-fat diet-induced colonic barrier dysfunction and inflammation in mice by regulating peroxisome proliferator-activated receptor expression and butyrate levels. Front Pharmacol. 2022; 13:930832.
Article32. Yang YN, Wang QC, Xu W, Yu J, Zhang H, Wu C. The berberine-enriched gut commensal Blautia producta ameliorates high-fat diet (HFD)-induced hyperlipidemia and stimulates liver LDLR expression. Biomed Pharmacother. 2022; 155:113749.
Article33. Wang FY, Liu JM, Luo HH, Liu AH, Jiang Y. Potential protective effects of Clostridium butyricum on experimental gastric ulcers in mice. World J Gastroenterol. 2015; 21:8340–51.
Article34. Liu J, Fu Y, Zhang H, Wang J, Zhu J, Wang Y, et al. The hepatoprotective effect of the probiotic Clostridium butyricum against carbon tetrachloride-induced acute liver damage in mice. Food Funct. 2017; 8:4042–52.
Article35. Jia L, Li D, Feng N, Shamoon M, Sun Z, Ding L, et al. Anti-diabetic effects of Clostridium butyricum CGMCC0313.1 through promoting the growth of gut butyrate-producing bacteria in type 2 diabetic mice. Sci Rep. 2017; 7:7046.
Article36. Jia L, Shan K, Pan LL, Feng N, Lv Z, Sun Y, et al. Clostridium butyricum CGMCC0313.1 protects against autoimmune diabetes by modulating intestinal immune homeostasis and inducing pancreatic regulatory T cells. Front Immunol. 2017; 8:1345.
Article37. Malik A, Morya RK, Saha S, Singh PK, Bhadada SK, Rana SV. Oxidative stress and inflammatory markers in type 2 diabetic patients. Eur J Clin Invest. 2020; 50:e13238.
Article38. Li H, Shi Y, Wang X, Li P, Zhang S, Wu T, et al. Piceatannol alleviates inflammation and oxidative stress via modulation of the Nrf2/HO-1 and NF-kB pathways in diabetic cardiomyopathy. Chem Biol Interact. 2019; 310:108754.39. Ichikawa T, Li J, Meyer CJ, Janicki JS, Hannink M, Cui T. Dihydro-CDDO-trifluoroethyl amide (dh404), a novel Nrf2 activator, suppresses oxidative stress in cardiomyocytes. PLoS One. 2009; 4:e8391.
Article40. Nguyen T, Sherratt PJ, Pickett CB. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol. 2003; 43:233–60.
Article41. Uruno A, Furusawa Y, Yagishita Y, Fukutomi T, Muramatsu H, Negishi T, et al. The Keap1-Nrf2 system prevents onset of diabetes mellitus. Mol Cell Biol. 2013; 33:2996–3010.
Article42. Di Marco E, Jha JC, Sharma A, Wilkinson-Berka JL, Jandeleit-Dahm KA, de Haan JB. Are reactive oxygen species still the basis for diabetic complications? Clin Sci (Lond). 2015; 129:199–216.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Clostridium butyricum on Gut Microbial Changes and Functional Profiles of Metabolism in High-fat Diet–fed Rats Depending on Age and Sex
- Gamma-tocopherol ameliorates hyperglycemia-induced hepatic inflammation associated with NLRP3 inflammasome in alloxan-induced diabetic mice
- Effects of Short Term Antioxidant Cocktail Supplementation on the Oxidative Stress and Inflammatory Response of Renal Inflammation in Diabetic Mice
- Similarities and differences between alpha-tocopherol and gamma-tocopherol in amelioration of inflammation, oxidative stress and pre-fibrosis in hyperglycemia induced acute kidney inflammation
- Molecular Mechanism of the Action of Clostridium botulinum Type B Neurotoxin