Ann Lab Med.
2024 May;44(3):253-261. 10.3343/alm.2023.0306.
Performance Evaluation of the Roche Cobas 5800 HBV and HCV Tests: Comparison of the 200 and 500 μL Protocols
- Affiliations
-
- 1Department of Laboratory Medicine, Hallym University Kangnam Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea
- 2Department of Laboratory Medicine, Hallym University Dongtan Sacred Heart Hospital, Hallym University College of Medicine, Hwaseong, Korea
- 3Department of Laboratory Medicine, Hallym University Sacred Heart Hospital, Hallym University College of Medicine, Anyang, Korea
- 4Department of Laboratory Medicine, Kangdong Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea
- KMID: 2555696
- DOI: http://doi.org/10.3343/alm.2023.0306
Abstract
- Background
Clinical management of patients infected with hepatitis B virus (HBV) or hepatitis C virus (HCV) relies on the viral load (VL). The Cobas 5800 system (Roche Diagnostics) can determine VLs in 200 and 500 µL samples, but the performance of each protocol has not been compared. We evaluated the performance of both protocols for the HBV and HCV tests.
Methods
Precision and linearity were verified using commercial panels. Probit analyses were used to determine limits of detection (LoDs). The results obtained with 336 samples were compared using the 200 and 500 µL protocols. Data from 6,737 retrospective HBV and 768 HCV samples were compared to estimate the effects of the different LoDs on the diagnostic results of the protocols. Correlations between protocols were tested with Spearman’s rank correlation coefficients (rho).
Results
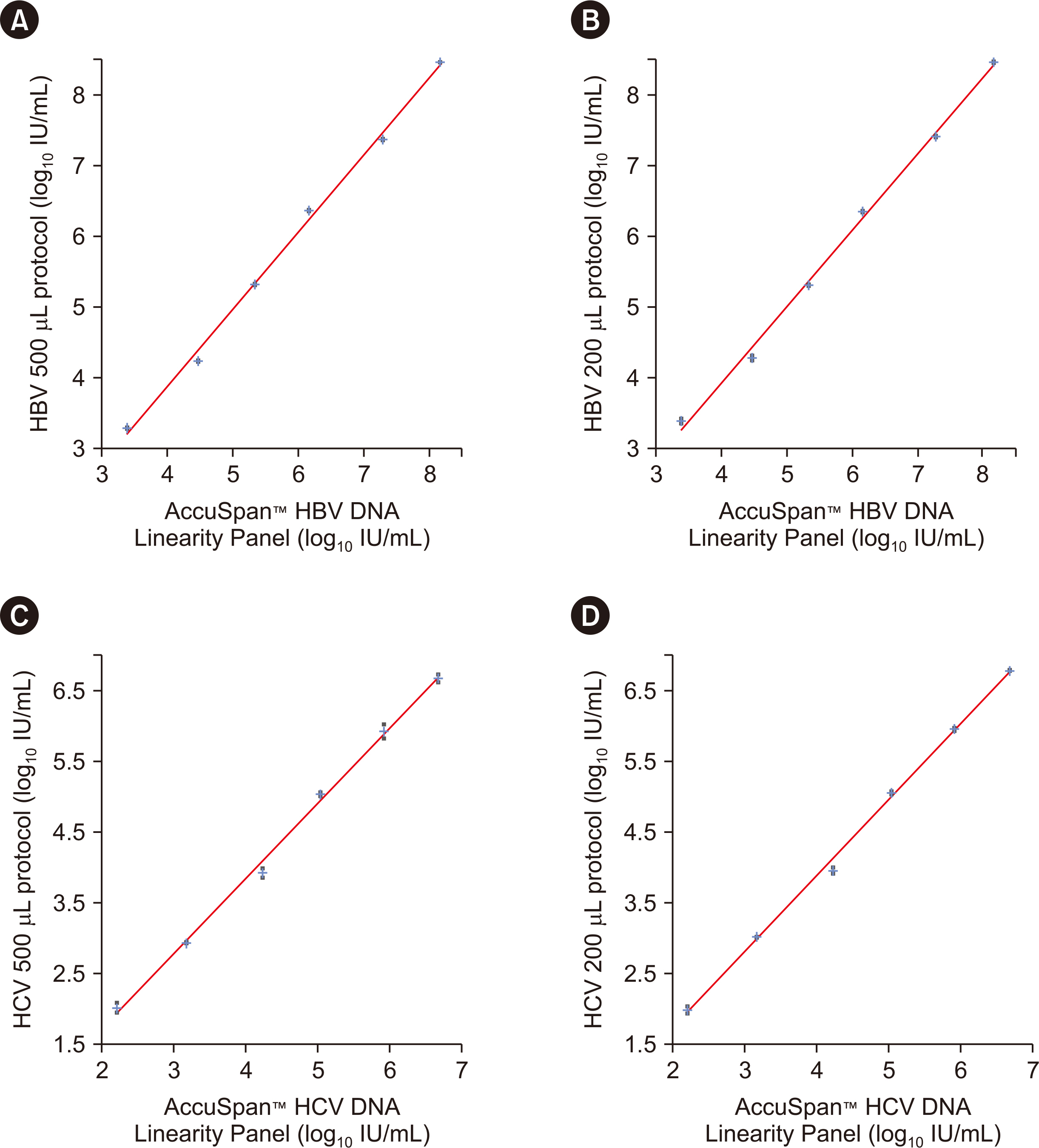
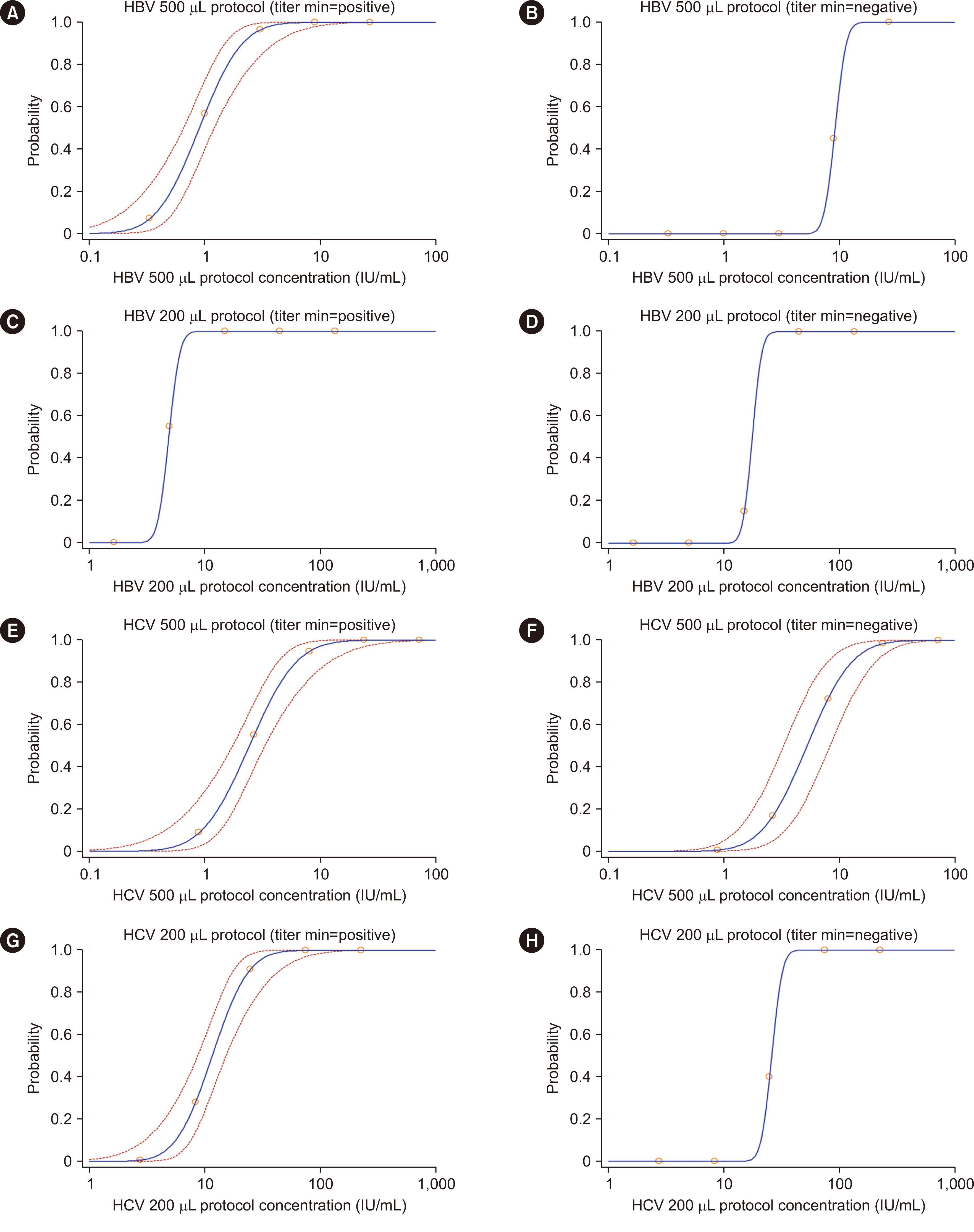
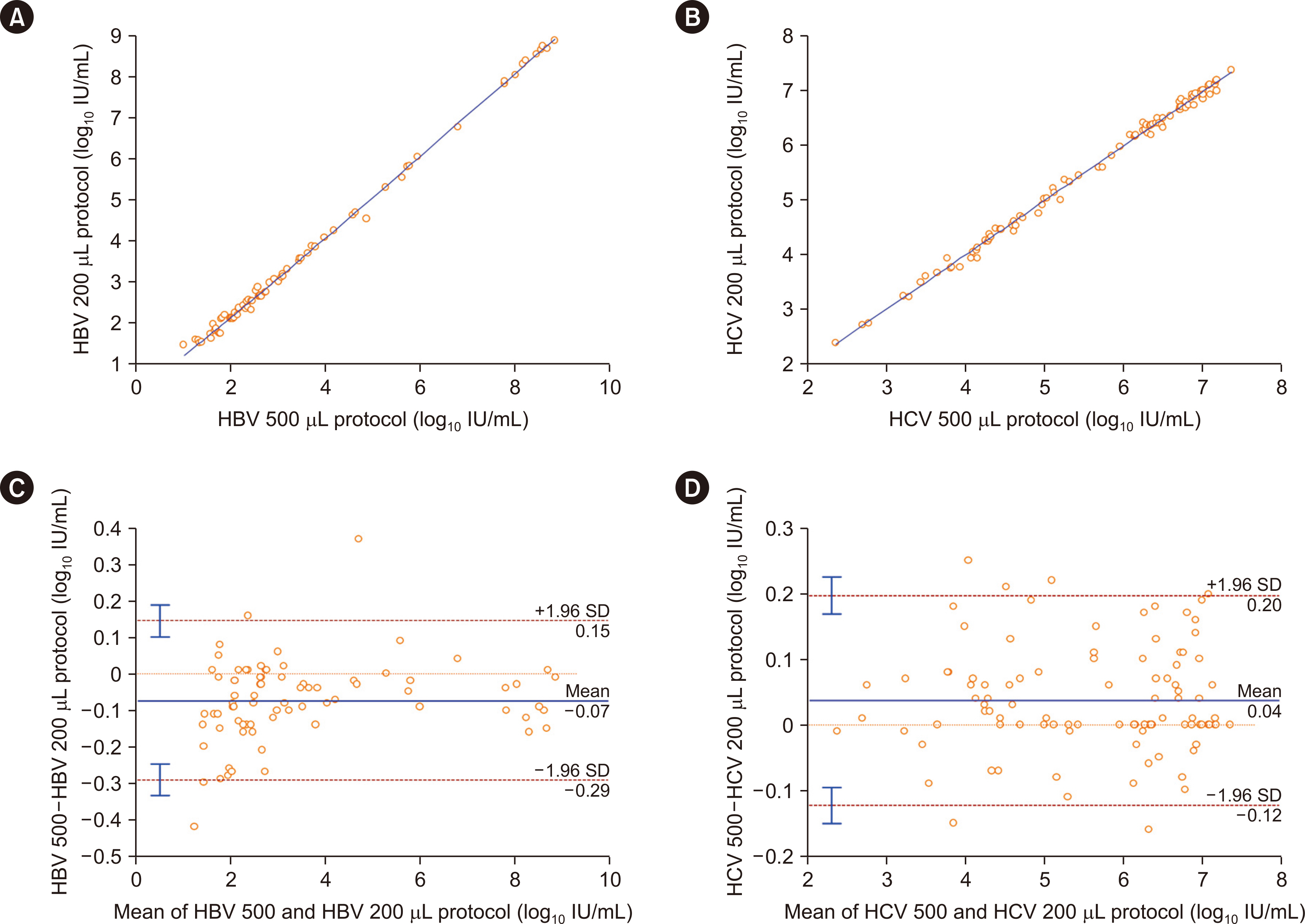
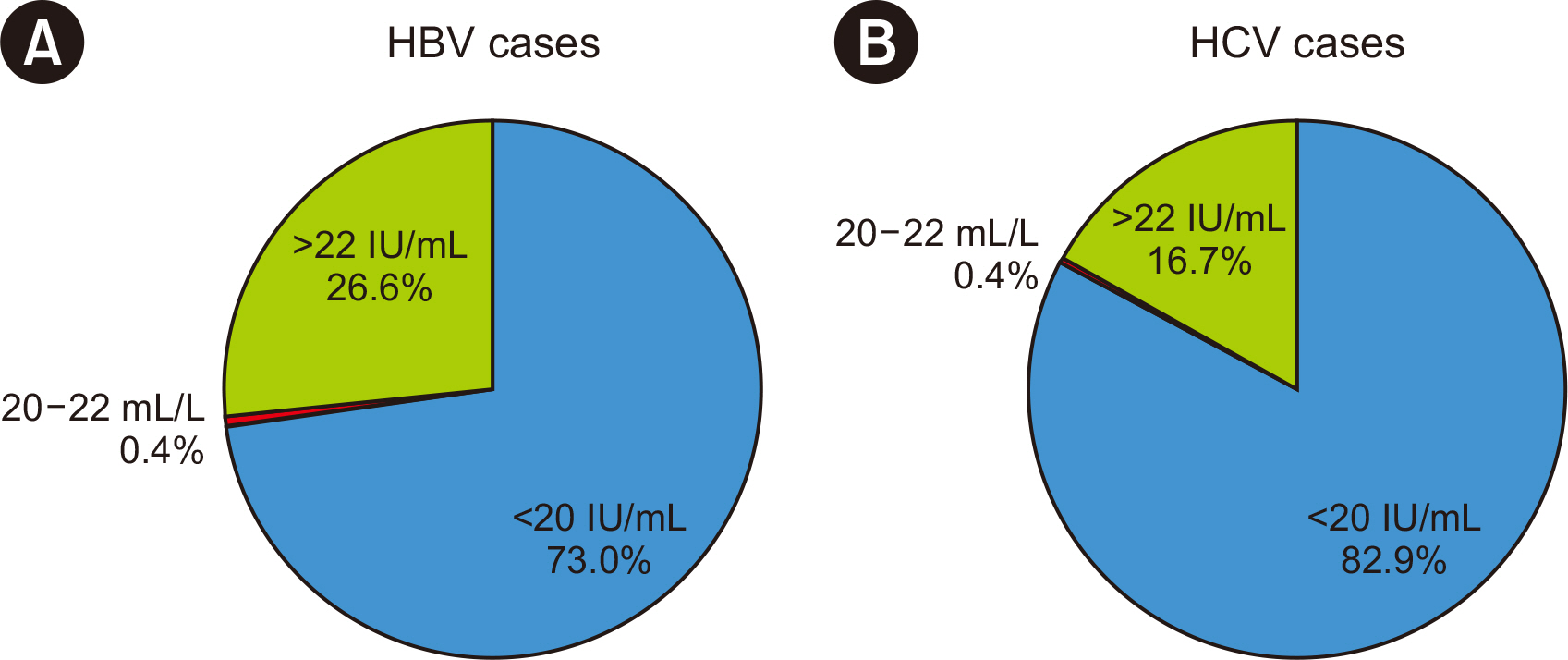
The precision and linearity of both protocols were verified. The LoDs for the 200 and 500 μL protocols were 6.5 and 2.7 IU/mL for HBV and 29.7 and 8.2 IU/mL for HCV, respectively. The agreement between the protocols ranged from 0.8 to 1.0. The results obtained with the HBV and HCV tests showed a strong correlation (rho = 0.994). Only 0.4% of HBV and 0.4% of HCV test results were affected by the LoDs of the 200 μL protocol.
Conclusions
The Cobas 5800 200 and 500 µL protocols for the HBV DNA and HCV RNA tests demonstrated excellent performance. These findings establish the 200 µL protocol as a new option for low-volume samples, especially for pediatric and difficult-to-bleed patients.
Keyword
Figure
Reference
-
References
1. World Health Organization. Guidelines on hepatitis B and C testing. http://apps.who.int/iris/bitstream/handle/10665/254621/9789241549981-eng.pdf. Updated on Feb 2017.2. American Association for the Study of Liver Diseases and Infectious Diseases Society of America. HCV guidance: recommendations for testing, managing, and treating hepatitis C. https://www.hcvguidelines.org/sites/default/files/full-guidance-pdf/AASLD-IDSA_HCVGuidance_October_05_2021.pdf. Updated on Oct 2021.3. European Association for the Study of the Liver. 2017; EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 67:370–98.4. European Association for the Study of the Liver. 2018; EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 69:461–511. DOI: 10.1016/j.jhep.2018.03.026. PMID: 29650333.5. Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. 2018; Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 67:1560–99. DOI: 10.1002/hep.29800. PMID: 29405329. PMCID: PMC5975958.6. Hur M, Park M, Moon HW, Choe WH, Lee CH. 2022; Comparison of non-invasive clinical algorithms for liver fibrosis in patients with chronic hepatitis B to reduce the need for liver biopsy: application of enhanced liver fibrosis and Mac-2 binding protein glycosylation isomer. Ann Lab Med. 42:249–57. DOI: 10.3343/alm.2022.42.2.249. PMID: 34635616. PMCID: PMC8548241.7. Andersson KL, Chung RT. 2009; Monitoring during and after antiviral therapy for hepatitis B. Hepatology. 49(S5):S166–73. DOI: 10.1002/hep.22899. PMID: 19399793. PMCID: PMC2679854.8. Braun P, Delgado R, Drago M, Fanti D, Fleury H, Izopet J, et al. 2017; A European multicientre study on the comparison of HBV viral loads between VERIS HBV assay and Roche COBAS® TAQMAN® HBV test, Abbott RealTime HBV assay, Siemens VERSANT HBV assay, and Qiagen Artus HBV RG kit. J Clin Virol. 95:76–83. DOI: 10.1016/j.jcv.2017.08.015. PMID: 28892764.9. Park Y, Roh J, Kim S. 2022; Performance evaluation of the Aptima assays in comparison with the cobas 6800 Assays for the detection of HIV-1, HBV, and HCV in clinical samples. Ann Lab Med. 42:447–56. DOI: 10.3343/alm.2022.42.4.447. PMID: 35177565. PMCID: PMC8859551.10. Tschäpe J, Cobernuss-Rahn A, Boyle S, Parkin N, LaBrot B, Aslam S, et al. 2022; Multisite performance evaluation of the Cobas 5800 System and comparison to the Cobas 6800/8800 systems for quantitative measurement of HBV, HCV, and HIV-1 viral load. Microbiol Spectr. 10:e0312522. DOI: 10.1128/spectrum.03125-22. PMID: 36314963. PMCID: PMC9769654.11. Tan NK, Carrington D, Pope CF. 2018; Verification of the Roche Cobas® 6800 PCR 200 µl and 500 µl protocols for the quantification of HIV-1 RNA, HBV DNA and HCV RNA and evaluation with COBAS® Ampliprep/COBAS® TaqMan® assays. J Med Microbiol. 67:1711–7. DOI: 10.1099/jmm.0.000838. PMID: 30325300.12. McHugh ML. 2012; Interrater reliability: the kappa statistic. Biochem Med (Zagreb). 22:276–82. DOI: 10.11613/BM.2012.031. PMID: 23092060.13. Schober P, Boer C, Schwarte LA. 2018; Correlation coefficients: appropriate use and interpretation. Anesth Analg. 126:1763–8. DOI: 10.1213/ANE.0000000000002864. PMID: 29481436.14. European Association for the Study of the Liver. 2012; EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol. 57:167–85. DOI: 10.1016/j.jhep.2012.02.010. PMID: 22436845.15. Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH, et al. 2016; AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 63:261–83. DOI: 10.1002/hep.28156. PMID: 26566064. PMCID: PMC5987259.16. Sidharthan S, Kohli A, Sims Z, Nelson A, Osinusi A, Masur H, et al. 2015; Utility of hepatitis C viral load monitoring on direct-acting antiviral therapy. Clin Infect Dis. 60:1743–51. DOI: 10.1093/cid/civ170. PMID: 25733369. PMCID: PMC4834854.17. Wirden M, Larrouy L, Mahjoub N, Todesco E, Damond F, Delagreverie H, et al. 2017; Multicenter comparison of the new Cobas 6800 system with Cobas Ampliprep/Cobas TaqMan and Abbott RealTime for the quantification of HIV, HBV and HCV viral load. J Clin Virol. 96:49–53. DOI: 10.1016/j.jcv.2017.09.007. PMID: 28950186.18. Baylis SA, Hanschmann KO, Schnierle BS, Trösemeier JH, Blümel J. Zika Virus Collaborative Study Group. 2017; Harmonization of nucleic acid testing for Zika virus: development of the 1st World Health Organization International Standard. Transfusion. 57:748–61. DOI: 10.1111/trf.14026. PMID: 28229462.19. Baylis SA, Wallace P, McCulloch E, Niesters HGM, Nübling CM. 2019; Standardization of nucleic acid tests: the approach of the World Health Organization. J Clin Microbiol. 57:e01056–18. DOI: 10.1128/JCM.01056-18. PMID: 30257900. PMCID: PMC6322456.20. Scholtès C, Hamilton AT, Plissonnier ML, Charre C, Scott B, Wang L, et al. Performance of the Cobas® HBV RNA automated investigational assay for the detection and quantification of circulating HBV RNA in chronic HBV patients. J Clin Virol. 2022; 150-151:105150. DOI: 10.1016/j.jcv.2022.105150. PMID: 35427860.21. Caliendo AM, Valsamakis A, Bremer JW, Ferreira-Gonzalez A, Granger S, Sabatini L, et al. 2011; Multilaboratory evaluation of real-time PCR tests for hepatitis B virus DNA quantification. J Clin Microbiol. 49:2854–8. DOI: 10.1128/JCM.00471-11. PMID: 21697326. PMCID: PMC3147738.22. Han MS, Park Y, Nah H, Kim HS. 2017; Comparison of the Qiagen Artus HBV QS-RGQ assay with the Roche COBAS AmpliPrep/COBAS TaqMan HBV assay for quantifying viral DNA in sera of chronic hepatitis B patients. Ann Lab Med. 37:248–53. DOI: 10.3343/alm.2017.37.3.248. PMID: 28224771. PMCID: PMC5339097.23. Ismail AM, Sivakumar J, Anantharam R, Dayalan S, Samuel P, Fletcher GJ, et al. 2011; Performance characteristics and comparison of Abbott and Artus real-time systems for hepatitis B virus DNA quantification. J Clin Microbiol. 49:3215–21. DOI: 10.1128/JCM.00915-11. PMID: 21795507. PMCID: PMC3165604.24. Yeh ML, Huang CF, Huang CI, Liu SF, Yang HL, Hsieh MY, et al. 2014; Abbott RealTime HBV assay is more sensitive in detection of low viral load and little impacted by drug resistant mutation in chronic hepatitis B patients under nucleot(s)ide analogues therapy. PLoS One. 9:e101790. DOI: 10.1371/journal.pone.0101790. PMID: 25000502. PMCID: PMC4085076.25. Vermehren J, Colucci G, Gohl P, Hamdi N, Abdelaziz AI, Karey U, et al. 2011; Development of a second version of the Cobas AmpliPrep/Cobas TaqMan hepatitis C virus quantitative test with improved genotype inclusivity. J Clin Microbiol. 49:3309–15. DOI: 10.1128/JCM.00602-11. PMID: 21752967. PMCID: PMC3165622.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Performance Evaluation of Aptima HBV and HCV Quant Assays in the Panther System
- Comparison of COBAS AmpliPrep/COBAS TaqMan HCV Qualitative Test v2.0 with COBAS AMPLICOR Hepatitis C Virus Test v2.0 for the Qualitative Detection of Hepatitis C Virus RNA in Korean Clinical Samples
- Performance Evaluation of the Roche-Hitachi cobas 8000 c702 Chemistry Autoanalyzer
- Comparison of the Diagnostic Performance of Elecsys Anti-HCV II and Elecsys and Vitros Anti-HCV Assays
- Report on the External Quality Assessment Scheme for Molecular Microbiology, Hepatitis Virus 1, 2 (2016–2021)