Korean J Physiol Pharmacol.
2024 May;28(3):239-252. 10.4196/kjpp.2024.28.3.239.
Dexmedetomidine alleviates blood-brain barrier disruption in rats after cerebral ischemia-reperfusion by suppressing JNK and p38 MAPK signaling
- Affiliations
-
- 1Department of Neurology, The First People's Hospital of Jiangxia District, Wuhan 430200, Hubei, China
- 2Department of Medicine, Soochow University, Suzhou 215006, Jiangsu, China
- 3Department of Vascular Neurosurgery, PLA Rocket Force Characteristic Medical Center, Beijing 100088, China
- KMID: 2555666
- DOI: http://doi.org/10.4196/kjpp.2024.28.3.239
Abstract
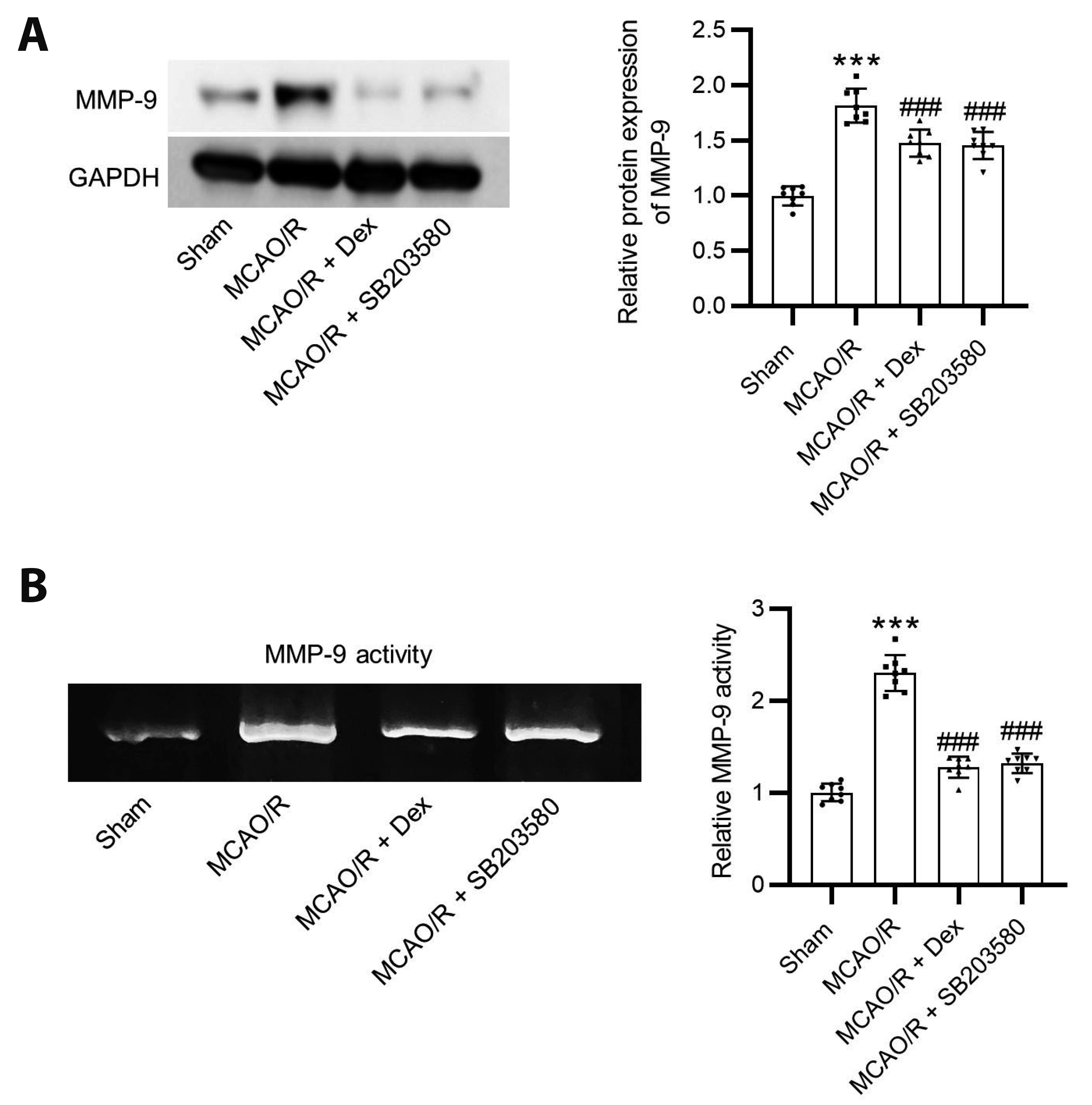
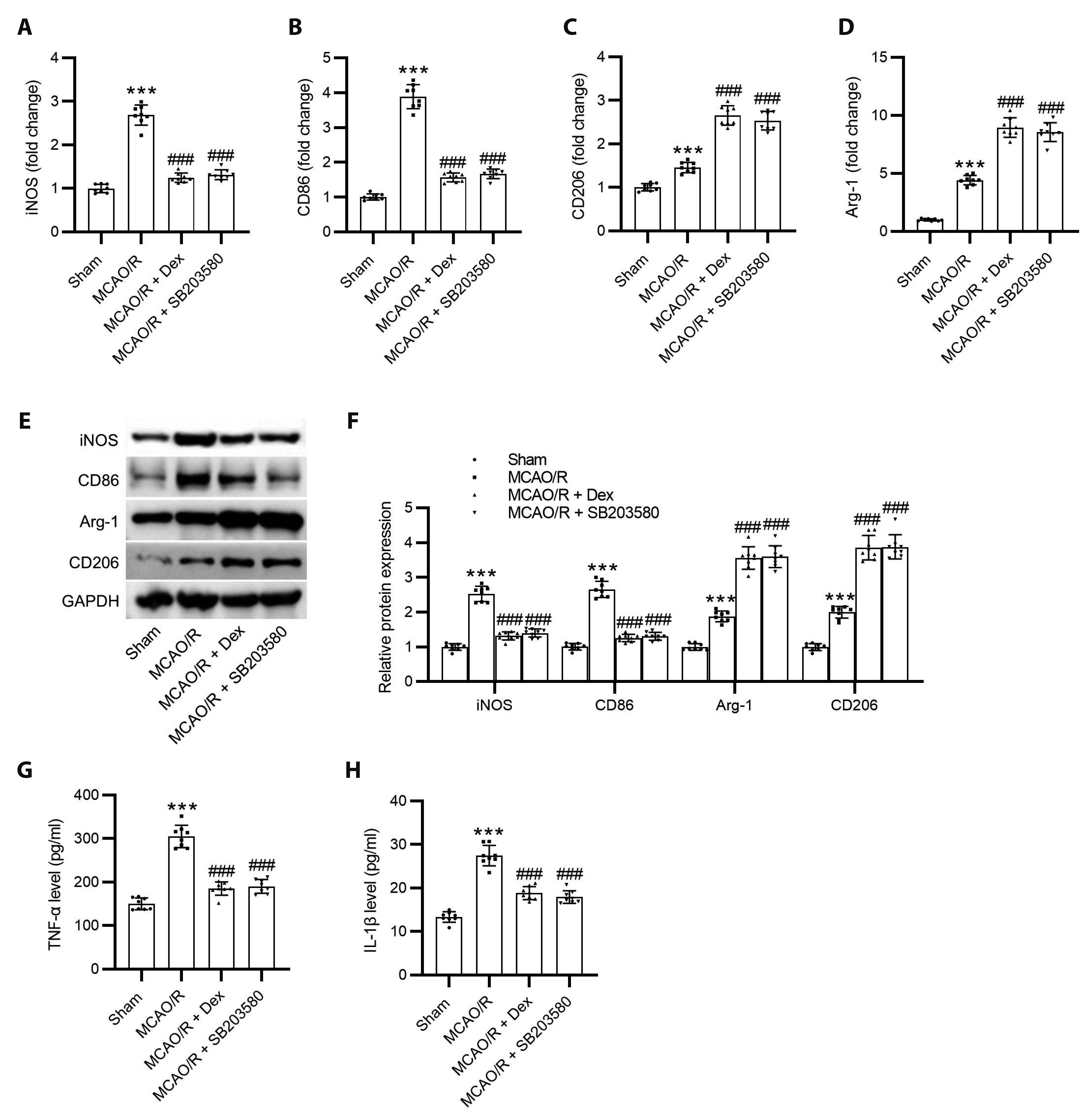
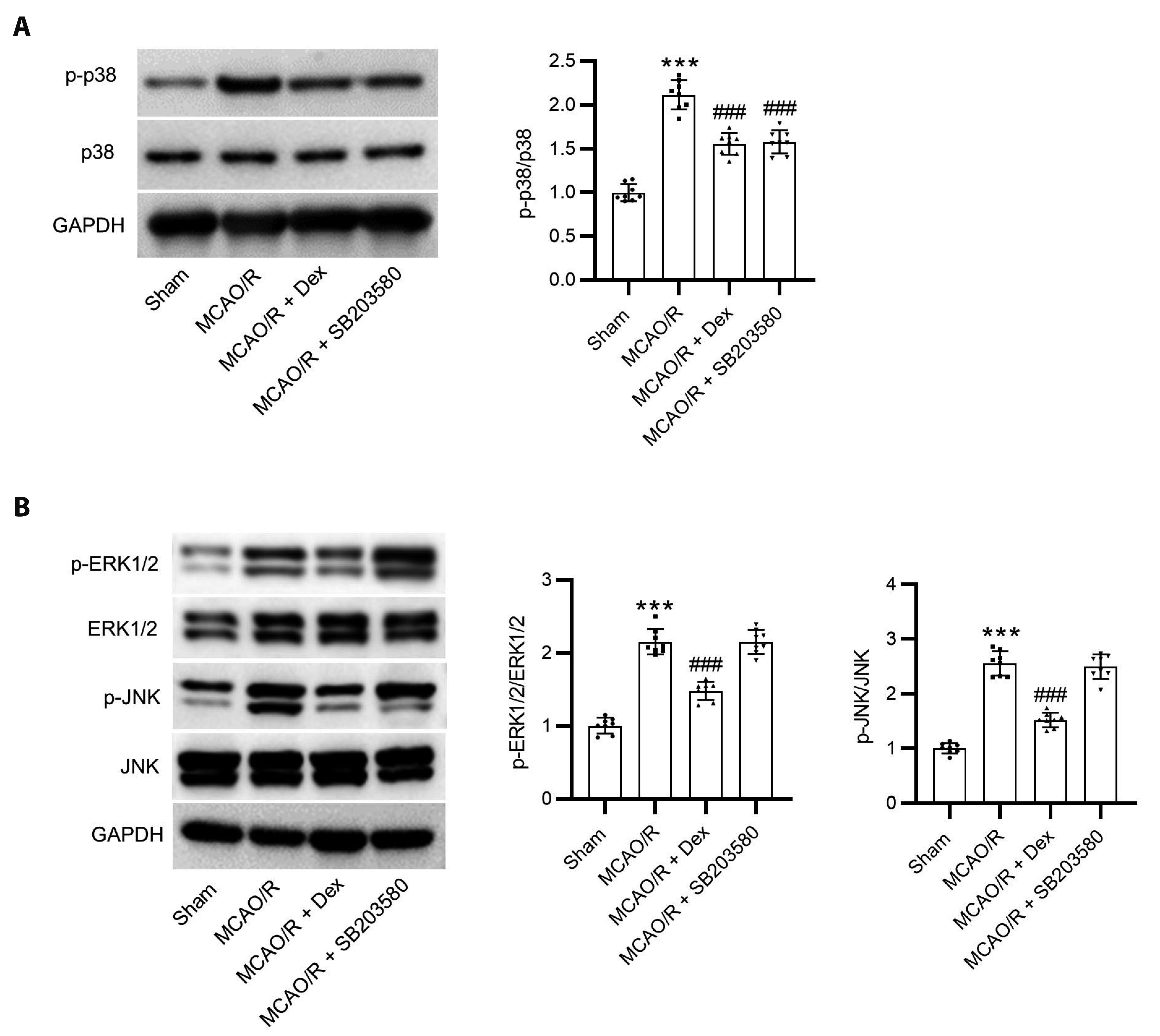
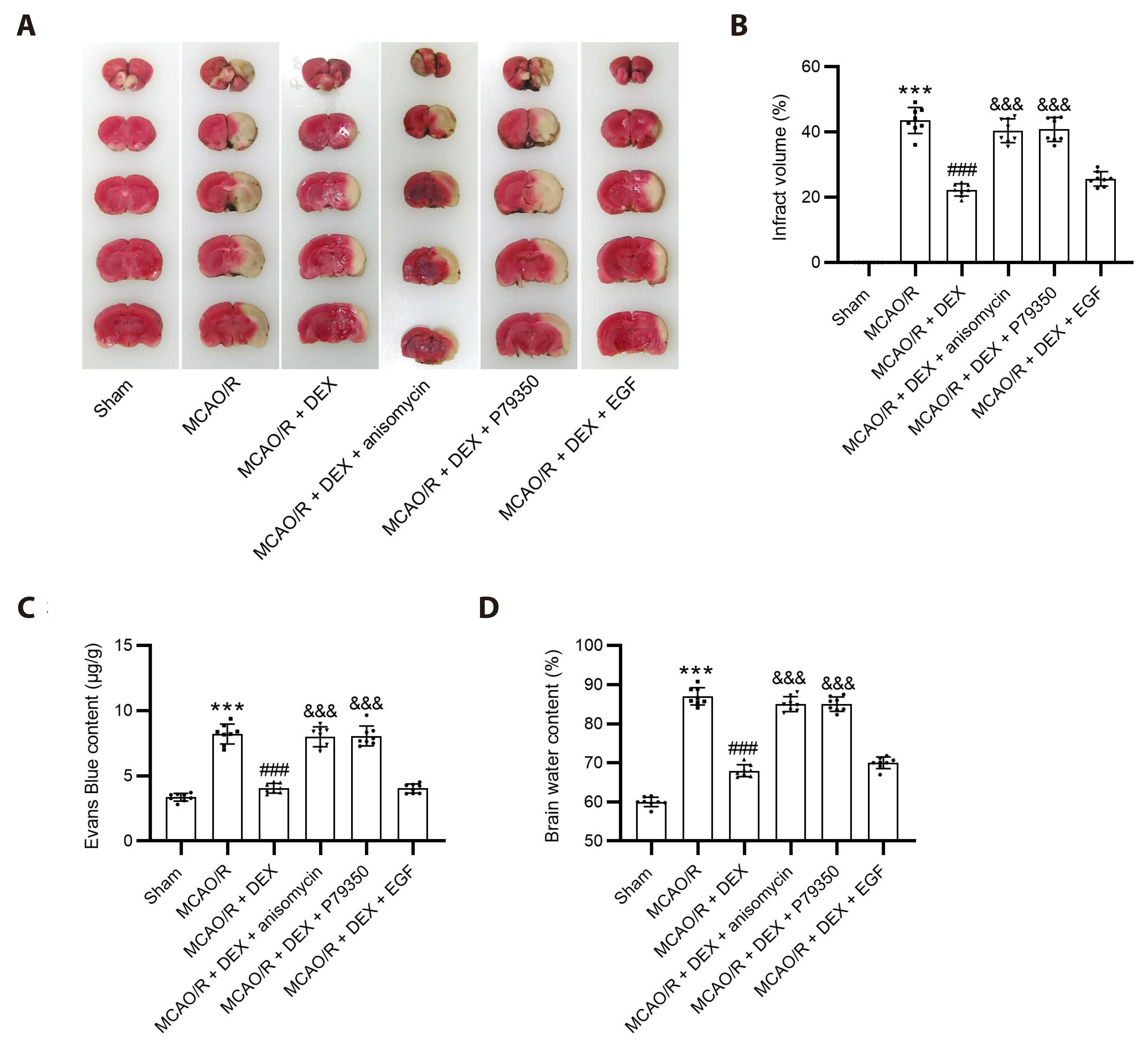
- Dexmedetomidine displays multiple mechanisms of neuroprotection in ameliorating ischemic brain injury. In this study, we explored the beneficial effects of dexmedetomidine on blood-brain barrier (BBB) integrity and neuroinflammation in cerebral ischemia/reperfusion injury. Sprague-Dawley rats were subjected to middle cerebral artery occlusion (MCAO) for 1.5 h and reperfusion for 24 h to establish a rat model of cerebral ischemia/reperfusion injury. Dexmedetomidine (9 µg/kg) was administered to rats 30 min after MCAO through intravenous injection, and SB203580 (a p38 MAPK inhibitor, 200 µg/kg) was injected intraperitoneally 30 min before MCAO. Brain damages were evaluated by 2,3,5-triphenyltetrazolium chloride staining, hematoxylin-eosin staining, Nissl staining, and brain water content assessment. BBB permeability was examined by Evans blue staining. Expression levels of claudin-5, zonula occludens-1, occludin, and matrix metalloproteinase-9 (MMP-9) as well as M1/M2 phenotypes-associated markers were assessed using immunofluorescence, RT-qPCR, Western blotting, and gelatin zymography. Enzyme-linked immunosorbent assay was used to examine inflammatory cytokine levels. We found that dexmedetomidine or SB203580 attenuated infarct volume, brain edema, BBB permeability, and neuroinflammation, and promoted M2 microglial polarization after cerebral ischemia/reperfusion injury. Increased MMP-9 activity by ischemia/reperfusion injury was inhibited by dexmedetomidine or SB203580. Dexmedetomidine inhibited the activation of the ERK, JNK, and p38 MAPK pathways. Moreover, activation of JNK or p38 MAPK reversed the protective effects of dexmedetomidine against ischemic brain injury. Overall, dexmedetomidine ameliorated brain injury by alleviating BBB permeability and promoting M2 polarization in experimental cerebral ischemia/reperfusion injury model by inhibiting the activation of JNK and p38 MAPK pathways.
Keyword
Figure
Reference
-
1. Miller JB, Merck LH, Wira CR, Meurer WJ, Schrock JW, Nomura JT, Siket MS, Madsen TE, Wright DW, Panagos PD, Lewandowski C. 2017; The advanced reperfusion era: implications for emergency systems of ischemic stroke care. Ann Emerg Med. 69:192–201. DOI: 10.1016/j.annemergmed.2016.06.042. PMID: 27600649.
Article2. Wilkins SS, Bourke P, Salam A, Akhtar N, DʼSouza A, Kamran S, Bhutta Z, Shuaib A. 2018; Functional stroke mimics: incidence and characteristics at a primary stroke center in the Middle East. Psychosom Med. 80:416–421. DOI: 10.1097/PSY.0000000000000563. PMID: 29394187. PMCID: PMC5991183.
Article3. Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley TJ, Wu TY, Brooks M, Simpson MA, Miteff F, Levi CR, Krause M, Harrington TJ, Faulder KC, Steinfort BS, Priglinger M, et al. 2015; ; EXTEND-IA Investigators. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 372:1009–1018. DOI: 10.1056/NEJMoa1414792. PMID: 25671797.
Article4. Barrett KM, Lal BK, Meschia JF. 2015; Stroke: advances in medical therapy and acute stroke intervention. Curr Cardiol Rep. 17:79. DOI: 10.1007/s11886-015-0637-1. PMID: 26277364.
Article5. Chapman SN, Mehndiratta P, Johansen MC, McMurry TL, Johnston KC, Southerland AM. 2014; Current perspectives on the use of intravenous recombinant tissue plasminogen activator (tPA) for treatment of acute ischemic stroke. Vasc Health Risk Manag. 10:75–87. DOI: 10.2147/VHRM.S39213. PMID: 24591838. PMCID: PMC3938499.6. Karatas H, Eun Jung J, Lo EH, van Leyen K. 2018; Inhibiting 12/15-lipoxygenase to treat acute stroke in permanent and tPA induced thrombolysis models. Brain Res. 1678:123–128. DOI: 10.1016/j.brainres.2017.10.024. PMID: 29079502. PMCID: PMC5714685.
Article7. Wang W, Li M, Chen Q, Wang J. 2015; Hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke: mechanisms, models, and biomarkers. Mol Neurobiol. 52:1572–1579. DOI: 10.1007/s12035-014-8952-x. PMID: 25367883. PMCID: PMC4418959.
Article8. Amani H, Mostafavi E, Alebouyeh MR, Arzaghi H, Akbarzadeh A, Pazoki-Toroudi H, Webster TJ. 2019; Would colloidal gold nanocarriers present an effective diagnosis or treatment for ischemic stroke? Int J Nanomedicine. 14:8013–8031. DOI: 10.2147/IJN.S210035. PMID: 31632015. PMCID: PMC6789974.9. Peluffo H, Unzueta U, Negro-Demontel ML, Xu Z, Váquez E, Ferrer-Miralles N, Villaverde A. 2015; BBB-targeting, protein-based nanomedicines for drug and nucleic acid delivery to the CNS. Biotechnol Adv. 33:277–287. DOI: 10.1016/j.biotechadv.2015.02.004. PMID: 25698504.
Article10. Merali Z, Huang K, Mikulis D, Silver F, Kassner A. 2017; Evolution of blood-brain-barrier permeability after acute ischemic stroke. PLoS One. 12:e0171558. DOI: 10.1371/journal.pone.0171558. PMID: 28207745. PMCID: PMC5313141.
Article11. Abdullahi W, Tripathi D, Ronaldson PT. 2018; Blood-brain barrier dysfunction in ischemic stroke: targeting tight junctions and transporters for vascular protection. Am J Physiol Cell Physiol. 315:C343–C356. DOI: 10.1152/ajpcell.00095.2018. PMID: 29949404. PMCID: PMC6171039.
Article12. Laskowitz DT, Kasner SE, Saver J, Remmel KS, Jauch EC. BRAIN Study Group. 2009; Clinical usefulness of a biomarker-based diagnostic test for acute stroke: the Biomarker Rapid Assessment in Ischemic Injury (BRAIN) study. Stroke. 40:77–85. DOI: 10.1161/STROKEAHA.108.516377. PMID: 18948614.
Article13. Picard K, St-Pierre MK, Vecchiarelli HA, Bordeleau M, Tremblay MÈ. 2021; Neuroendocrine, neuroinflammatory and pathological outcomes of chronic stress: a story of microglial remodeling. Neurochem Int. 145:104987. DOI: 10.1016/j.neuint.2021.104987. PMID: 33587954.
Article14. Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. 2011; Physiology of microglia. Physiol Rev. 91:461–553. DOI: 10.1152/physrev.00011.2010. PMID: 21527731.
Article15. Borlongan CV. 2009; Cell therapy for stroke: remaining issues to address before embarking on clinical trials. Stroke. 40(3 Suppl):S146–S148. DOI: 10.1161/STROKEAHA.108.533091. PMCID: PMC4810678.16. Franco R, Fernández-Suárez D. 2015; Alternatively activated microglia and macrophages in the central nervous system. Prog Neurobiol. 131:65–86. DOI: 10.1016/j.pneurobio.2015.05.003. PMID: 26067058.
Article17. Thurgur H, Pinteaux E. 2019; Microglia in the neurovascular unit: blood-brain barrier-microglia interactions after central nervous system disorders. Neuroscience. 405:55–67. DOI: 10.1016/j.neuroscience.2018.06.046. PMID: 31007172.
Article18. Dhillon AS, Hagan S, Rath O, Kolch W. 2007; MAP kinase signalling pathways in cancer. Oncogene. 26:3279–3290. DOI: 10.1038/sj.onc.1210421. PMID: 17496922.
Article19. Jiang M, Li J, Peng Q, Liu Y, Liu W, Luo C, Peng J, Li J, Yung KK, Mo Z. 2014; Neuroprotective effects of bilobalide on cerebral ischemia and reperfusion injury are associated with inhibition of pro-inflammatory mediator production and down-regulation of JNK1/2 and p38 MAPK activation. J Neuroinflammation. 11:167. DOI: 10.1186/s12974-014-0167-6. PMID: 25256700. PMCID: PMC4189683.
Article20. Wang L, Li Z, Zhang X, Wang S, Zhu C, Miao J, Chen L, Cui L, Qiao H. 2014; Protective effect of shikonin in experimental ischemic stroke: attenuated TLR4, p-p38MAPK, NF-κB, TNF-α and MMP-9 expression, up-regulated claudin-5 expression, ameliorated BBB permeability. Neurochem Res. 39:97–106. DOI: 10.1007/s11064-013-1194-x. PMID: 24248858.
Article21. Pfeilschifter W, Czech B, Hoffmann BP, Sujak M, Kahles T, Steinmetz H, Neumann-Haefelin T, Pfeilschifter J. 2010; Pyrrolidine dithiocarbamate activates p38 MAPK and protects brain endothelial cells from apoptosis: a mechanism for the protective effect in stroke? Neurochem Res. 35:1391–1401. DOI: 10.1007/s11064-010-0197-0. PMID: 20514517.
Article22. Cheng CY, Lin JG, Tang NY, Kao ST, Hsieh CL. 2015; Electroacupuncture at different frequencies (5Hz and 25Hz) ameliorates cerebral ischemia-reperfusion injury in rats: possible involvement of p38 MAPK-mediated anti-apoptotic signaling pathways. BMC Complement Altern Med. 15:241. DOI: 10.1186/s12906-015-0752-y. PMID: 26187498. PMCID: PMC4506591.
Article23. Wang J, Bai T, Wang N, Li H, Guo X. 2020; Neuroprotective potential of imatinib in global ischemia-reperfusion-induced cerebral injury: possible role of Janus-activated kinase 2/signal transducer and activator of transcription 3 and connexin 43. Korean J Physiol Pharmacol. 24:11–18. DOI: 10.4196/kjpp.2020.24.1.11. PMID: 31908570. PMCID: PMC6940502.
Article24. Shin MC, Lee TK, Lee JC, Kim HI, Park CW, Cho JH, Kim DW, Ahn JH, Won MH, Lee CH. 2022; Therapeutic effects of stiripentol against ischemia-reperfusion injury in gerbils focusing on cognitive deficit, neuronal death, astrocyte damage and blood brain barrier leakage in the hippocampus. Korean J Physiol Pharmacol. 26:47–57. DOI: 10.4196/kjpp.2022.26.1.47. PMID: 34965995. PMCID: PMC8723979.
Article25. Gao Y, Yin H, Zhang Y, Dong Y, Yang F, Wu X, Liu H. 2019; Dexmedetomidine protects hippocampal neurons against hypoxia/reoxygenation-induced apoptosis through activation HIF-1α/p53 signaling. Life Sci. 232:116611. DOI: 10.1016/j.lfs.2019.116611. PMID: 31260683.
Article26. Zhou W, Zhang Y, Jiao Y, Yin W, Dong H, Xu S, Tang D, Jiang J, Shao J, Wang Z, Yu W. 2021; Dexmedetomidine maintains blood-brain barrier integrity by inhibiting Drp1-related endothelial mitochondrial dysfunction in ischemic stroke. Acta Biochim Biophys Sin (Shanghai). 53:1177–1188. DOI: 10.1093/abbs/gmab092. PMID: 34244711.
Article27. Zhao Q, Yu S, Ling Y, Hao S, Liu J. 2021; The protective effects of dexmedetomidine against hypoxia/reoxygenation-induced inflammatory injury and permeability in brain endothelial cells mediated by Sigma-1 receptor. ACS Chem Neurosci. 12:1940–1947. DOI: 10.1021/acschemneuro.1c00032. PMID: 34014076.
Article28. Tian M, Wang W, Wang K, Jin P, Lenahan C, Wang Y, Tan J, Wen H, Deng S, Zhao F, Gong Y. 2021; Dexmedetomidine alleviates cognitive impairment by reducing blood-brain barrier interruption and neuroinflammation via regulating Th1/Th2/Th17 polarization in an experimental sepsis model of mice. Int Immunopharmacol. 101:108332. DOI: 10.1016/j.intimp.2021.108332. PMID: 34785141.
Article29. Yin D, Zhou S, Xu X, Gao W, Li F, Ma Y, Sun D, Wu Y, Guo Q, Liu H, Han L, Wang Z, Wang Y, Zhang J. 2018; Dexmedetomidine attenuated early brain injury in rats with subarachnoid haemorrhage by suppressing the inflammatory response: The TLR4/NF-κB pathway and the NLRP3 inflammasome may be involved in the mechanism. Brain Res. 1698:1–10. DOI: 10.1016/j.brainres.2018.05.040. PMID: 29842860.
Article30. Qiu Z, Lu P, Wang K, Zhao X, Li Q, Wen J, Zhang H, Li R, Wei H, Lv Y, Zhang S, Zhang P. 2020; Dexmedetomidine inhibits neuroinflammation by altering microglial M1/M2 polarization through MAPK/ERK pathway. Neurochem Res. 45:345–353. DOI: 10.1007/s11064-019-02922-1. PMID: 31823113.
Article31. Liu XM, Chen QH, Hu Q, Liu Z, Wu Q, Liang SS, Zhang HG, Zhang Q, Zhang XK. 2020; Dexmedetomidine protects intestinal ischemia-reperfusion injury via inhibiting p38 MAPK cascades. Exp Mol Pathol. 115:104444. DOI: 10.1016/j.yexmp.2020.104444. PMID: 32335082.
Article32. Yeda X, Shaoqing L, Yayi H, Bo Z, Huaxin W, Hong C, Zhongyuan X. 2017; Dexmedetomidine protects against renal ischemia and reperfusion injury by inhibiting the P38-MAPK/TXNIP signaling activation in streptozotocin induced diabetic rats. Acta Cir Bras. 32:429–439. DOI: 10.1590/s0102-865020170060000003. PMID: 28700004.
Article33. Jiang L, Li L, Shen J, Qi Z, Guo L. 2014; Effect of dexmedetomidine on lung ischemiareperfusion injury. Mol Med Rep. 9:419–426. DOI: 10.3892/mmr.2013.1867. PMID: 24345905. PMCID: PMC3896524.
Article34. Lempiäinen J, Finckenberg P, Mervaala EE, Storvik M, Kaivola J, Lindstedt K, Levijoki J, Mervaala EM. 2014; Dexmedetomidine preconditioning ameliorates kidney ischemia-reperfusion injury. Pharmacol Res Perspect. 2:e00045. DOI: 10.1002/prp2.45. PMID: 25505591. PMCID: PMC4186414.
Article35. Zheng X, Cai X, Ye F, Li Y, Wang Q, Zuo Z, Huang W, Wang Z. 2020; Perioperative dexmedetomidine attenuates brain ischemia reperfusion injury possibly via up-regulation of astrocyte Connexin 43. BMC Anesthesiol. 20:299. DOI: 10.1186/s12871-020-01211-7. PMID: 33287729. PMCID: PMC7722427.
Article36. Luo C, Ouyang MW, Fang YY, Li SJ, Zhou Q, Fan J, Qin ZS, Tao T. 2017; Dexmedetomidine protects mouse brain from ischemia-reperfusion injury via inhibiting neuronal autophagy through up-regulating HIF-1α. Front Cell Neurosci. 11:197. DOI: 10.3389/fncel.2017.00197. PMID: 28729825. PMCID: PMC5498477.
Article37. Longa EZ, Weinstein PR, Carlson S, Cummins R. 1989; Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 20:84–91. DOI: 10.1161/01.STR.20.1.84. PMID: 2643202.
Article38. Guo Q, Ma M, Yu H, Han Y, Zhang D. 2023; Dexmedetomidine enables copper homeostasis in cerebral ischemia/reperfusion via ferredoxin 1. Ann Med. 55:2209735. DOI: 10.1080/07853890.2023.2209735. PMID: 37162502. PMCID: PMC10173798.
Article39. Yin JW, Li J, Ren YM, Li Y, Wang RX, Wang S, Zuo YX. 2021; Dexmedetomidine and Netrin-1 combination therapy inhibits endoplasmic reticulum stress by regulating the ERK5/MEF2A pathway to attenuate cerebral ischemia injury. Front Neurosci. 15:641345. DOI: 10.3389/fnins.2021.641345. PMID: 33584197. PMCID: PMC7876398.
Article40. Yamashita S, Hirata T, Mizukami Y, Cui YJ, Fukuda S, Ishida K, Matsumoto M, Sakabe T. 2009; Repeated preconditioning with hyperbaric oxygen induces neuroprotection against forebrain ischemia via suppression of p38 mitogen activated protein kinase. Brain Res. 1301:171–179. DOI: 10.1016/j.brainres.2009.08.096. PMID: 19747454.
Article41. Han X, Mao Z, Wang S, Xin Y, Li P, Maharjan S, Zhang B. 2020; GYY4137 protects against MCAO via p38 MAPK mediated anti-apoptotic signaling pathways in rats. Brain Res Bull. 158:59–65. DOI: 10.1016/j.brainresbull.2020.02.015. PMID: 32114001.
Article42. Thored P, Wood J, Arvidsson A, Cammenga J, Kokaia Z, Lindvall O. 2007; Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke. 38:3032–3039. DOI: 10.1161/STROKEAHA.107.488445. PMID: 17901386.
Article43. Chen XY, Yang LP, Zheng YL, Li YX, Zhong DL, Jin RJ, Li J. 2022; Electroacupuncture attenuated phenotype transformation of vascular smooth muscle cells via PI3K/Akt and MAPK signaling pathways in spontaneous hypertensive rats. Chin J Integr Med. 28:357–365. DOI: 10.1007/s11655-021-2883-y. PMID: 34839455.
Article44. Zeng JJ, Shi HQ, Ren FF, Zhao XS, Chen QY, Wang DJ, Wu LP, Chu MP, Lai TF, Li L. 2023; Notoginsenoside R1 protects against myocardial ischemia/reperfusion injury in mice via suppressing TAK1-JNK/p38 signaling. Acta Pharmacol Sin. 44:1366–1379. DOI: 10.1038/s41401-023-01057-y. PMID: 36721009.
Article45. Zhao TC, Zhang L, Liu JT, Guo TL. 2012; Disruption of Nox2 and TNFRp55/p75 eliminates cardioprotection induced by anisomycin. Am J Physiol Heart Circ Physiol. 303:H1263–H1272. DOI: 10.1152/ajpheart.00306.2012. PMID: 22982779. PMCID: PMC3517639.
Article46. Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. 1986; Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 17:472–476. DOI: 10.1161/01.STR.17.3.472. PMID: 3715945.
Article47. Tsubokawa T, Jadhav V, Solaroglu I, Shiokawa Y, Konishi Y, Zhang JH. 2007; Lecithinized superoxide dismutase improves outcomes and attenuates focal cerebral ischemic injury via antiapoptotic mechanisms in rats. Stroke. 38:1057–1062. DOI: 10.1161/01.STR.0000257978.70312.1d. PMID: 17272760.
Article48. Lu XY, Wang HD, Xu JG, Ding K, Li T. 2015; Deletion of Nrf2 exacerbates oxidative stress after traumatic brain injury in mice. Cell Mol Neurobiol. 35:713–721. DOI: 10.1007/s10571-015-0167-9. PMID: 25732597.
Article49. Livak KJ, Schmittgen TD. 2001; Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. DOI: 10.1006/meth.2001.1262. PMID: 11846609.
Article50. Yue Y, Zhao H, Yue Y, Zhang Y, Wei W. 2020; Downregulation of Microrna-421 relieves cerebral ischemia/reperfusion injuries: involvement of anti-apoptotic and antioxidant activities. Neuromolecular Med. 22:411–419. DOI: 10.1007/s12017-020-08600-8. PMID: 32385800.
Article51. Liu Y, Wang D, Wang H, Qu Y, Xiao X, Zhu Y. 2014; The protective effect of HET0016 on brain edema and blood-brain barrier dysfunction after cerebral ischemia/reperfusion. Brain Res. 1544:45–53. DOI: 10.1016/j.brainres.2013.11.031. PMID: 24316243.
Article52. Unchiti K, Leurcharusmee P, Samerchua A, Pipanmekaporn T, Chattipakorn N, Chattipakorn SC. 2021; The potential role of dexmedetomidine on neuroprotection and its possible mechanisms: evidence from in vitro and in vivo studies. Eur J Neurosci. 54:7006–7047. DOI: 10.1111/ejn.15474. PMID: 34561931.
Article53. Zeng X, Wang H, Xing X, Wang Q, Li W. 2016; Dexmedetomidine protects against transient global cerebral ischemia/reperfusion induced oxidative stress and inflammation in diabetic rats. PLoS One. 11:e0151620. DOI: 10.1371/journal.pone.0151620. PMID: 26982373. PMCID: PMC4794239.
Article54. Yu W, Lyu J, Jia L, Sheng M, Yu H, Du H. 2020; Dexmedetomidine ameliorates hippocampus injury and cognitive dysfunction induced by hepatic ischemia/reperfusion by activating SIRT3-mediated mitophagy and inhibiting activation of the NLRP3 inflammasome in young rats. Oxid Med Cell Longev. 2020:7385458. Erratum. DOI: 10.1155/2020/7385458. PMID: 34493950. PMCID: PMC8418694.
Article55. Ren X, Ma H, Zuo Z. 2016; Dexmedetomidine postconditioning reduces brain injury after brain hypoxia-ischemia in neonatal rats. J Neuroimmune Pharmacol. 11:238–247. DOI: 10.1007/s11481-016-9658-9. PMID: 26932203.
Article56. Kamada H, Yu F, Nito C, Chan PH. 2007; Influence of hyperglycemia on oxidative stress and matrix metalloproteinase-9 activation after focal cerebral ischemia/reperfusion in rats: relation to blood-brain barrier dysfunction. Stroke. 38:1044–1049. DOI: 10.1161/01.STR.0000258041.75739.cb. PMID: 17272778. PMCID: PMC1828129.
Article57. Song HL, Zhang SB. 2019; Therapeutic effect of dexmedetomidine on intracerebral hemorrhage via regulating NLRP3. Eur Rev Med Pharmacol Sci. 23:2612–2619.58. Alluri H, Peddaboina CS, Tharakan B. 2024; Determination of tight junction integrity in brain endothelial cells based on tight junction protein expression. Methods Mol Biol. 2711:235–240. DOI: 10.1007/978-1-0716-3429-5_19. PMID: 37776462.
Article59. Knowland D, Arac A, Sekiguchi KJ, Hsu M, Lutz SE, Perrino J, Steinberg GK, Barres BA, Nimmerjahn A, Agalliu D. 2014; Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke. Neuron. 82:603–617. DOI: 10.1016/j.neuron.2014.03.003. PMID: 24746419. PMCID: PMC4016169.
Article60. Romanic AM, White RF, Arleth AJ, Ohlstein EH, Barone FC. 1998; Matrix metalloproteinase expression increases after cerebral focal ischemia in rats: inhibition of matrix metalloproteinase-9 reduces infarct size. Stroke. 29:1020–1030. DOI: 10.1161/01.STR.29.5.1020. PMID: 9596253.
Article61. Huang W, Chen L, Zhang B, Park M, Toborek M. 2014; PPAR agonist-mediated protection against HIV Tat-induced cerebrovascular toxicity is enhanced in MMP-9-deficient mice. J Cereb Blood Flow Metab. 34:646–653. DOI: 10.1038/jcbfm.2013.240. PMID: 24424383. PMCID: PMC3982084.
Article62. Fujimura M, Gasche Y, Morita-Fujimura Y, Massengale J, Kawase M, Chan PH. 1999; Early appearance of activated matrix metalloproteinase-9 and blood-brain barrier disruption in mice after focal cerebral ischemia and reperfusion. Brain Res. 842:92–100. DOI: 10.1016/S0006-8993(99)01843-0. PMID: 10526099.
Article63. Xin X, Chen J, Hua W, Wang H. 2021; Intraoperative dexmedetomidine for prevention of postoperative delirium in elderly patients with mild cognitive impairment. Int J Geriatr Psychiatry. 36:143–151. DOI: 10.1002/gps.5406. PMID: 33411362.
Article64. Fang B, Li XQ, Bi B, Tan WF, Liu G, Zhang Y, Ma H. 2015; Dexmedetomidine attenuates blood-spinal cord barrier disruption induced by spinal cord ischemia reperfusion injury in rats. Cell Physiol Biochem. 36:373–383. DOI: 10.1159/000430107. PMID: 25967975.
Article65. Huang X, Hussain B, Chang J. 2021; Peripheral inflammation and blood-brain barrier disruption: effects and mechanisms. CNS Neurosci Ther. 27:36–47. DOI: 10.1111/cns.13569. PMID: 33381913. PMCID: PMC7804893.
Article66. Su Y, Gao J, Kaur P, Wang Z. 2020; Neutrophils and macrophages as targets for development of nanotherapeutics in inflammatory diseases. Pharmaceutics. 12:1222. DOI: 10.3390/pharmaceutics12121222. PMID: 33348630. PMCID: PMC7766591.
Article67. Kanazawa M, Ninomiya I, Hatakeyama M, Takahashi T, Shimohata T. 2017; Microglia and monocytes/macrophages polarization reveal novel therapeutic mechanism against stroke. Int J Mol Sci. 18:2135. DOI: 10.3390/ijms18102135. PMID: 29027964. PMCID: PMC5666817.
Article68. Zhu Y, Li S, Liu J, Wen Q, Yu J, Yu L, Xie K. 2019; Role of JNK signaling pathway in dexmedetomidine post-conditioning-induced reduction of the inflammatory response and autophagy effect of focal cerebral ischemia reperfusion injury in rats. Inflammation. 42:2181–2191. DOI: 10.1007/s10753-019-01082-2. PMID: 31446520.
Article69. Wang J, Xin Y, Chu T, Liu C, Xu A. 2022; Dexmedetomidine attenuates perioperative neurocognitive disorders by suppressing hippocampal neuroinflammation and HMGB1/RAGE/NF-κB signaling pathway. Biomed Pharmacother. 150:113006. DOI: 10.1016/j.biopha.2022.113006. PMID: 35486975.
Article70. Wang N, Nie H, Zhang Y, Han H, Wang S, Liu W, Tian K. 2022; Dexmedetomidine exerts cerebral protective effects against cerebral ischemic injury by promoting the polarization of M2 microglia via the Nrf2/HO-1/NLRP3 pathway. Inflamm Res. 71:93–106. DOI: 10.1007/s00011-021-01515-5. PMID: 34767031.
Article71. Lahiani A, Brand-Yavin A, Yavin E, Lazarovici P. 2018; Neuroprotective effects of bioactive compounds and MAPK pathway modulation in "ischemia"-stressed PC12 pheochromocytoma cells. Brain Sci. 8:32. DOI: 10.3390/brainsci8020032. PMID: 29419806. PMCID: PMC5836051.
Article72. Sun C, Qi R, Wang L, Yan J, Wang Y. 2012; p38 MAPK regulates calcium signal-mediated lipid accumulation through changing VDR expression in primary preadipocytes of mice. Mol Biol Rep. 39:3179–3184. DOI: 10.1007/s11033-011-1084-8. PMID: 21701827.
Article73. Godl K, Wissing J, Kurtenbach A, Habenberger P, Blencke S, Gutbrod H, Salassidis K, Stein-Gerlach M, Missio A, Cotten M, Daub H. 2003; An efficient proteomics method to identify the cellular targets of protein kinase inhibitors. Proc Natl Acad Sci U S A. 100:15434–15439. DOI: 10.1073/pnas.2535024100. PMID: 14668439. PMCID: PMC307585.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Rapid Disruption of Cellular Integrity of Zinc-treated Astroglia Is Regulated by p38 MAPK and Ca(2+)-dependent Mechanisms
- Neuroprotection of Dexmedetomidine against Cerebral Ischemia-Reperfusion Injury in Rats: Involved in Inhibition of NF-κB and Inflammation Response
- Contributions of HO-1-Dependent MAPK to Regulating Intestinal Barrier Disruption
- Protective Effect of Sauchinone Against Regional Myocardial Ischemia/Reperfusion Injury: Inhibition of p38 MAPK and JNK Death Signaling Pathways
- Effect of Mild Hypothermia on the Mitogen Activated Protein Kinases in Experimental Stroke