Acute Crit Care.
2024 Feb;39(1):194-198. 10.4266/acc.2021.01158.
Successful extracorporeal membrane oxygenation treatment of catecholamine-induced cardiomyopathy-associated pheochromocytoma: a case report
- Affiliations
-
- 1Department of Internal Medicine, Chungbuk National University Hospital, Cheongju, Korea
- 2Regional Cardiovascular Disease Center, Chungbuk National University Hospital, Cheongju, Korea
- 3Division of Cardiology, Department of Internal Medicine, Chungbuk National University College of Medicine, Cheongju, Korea
- KMID: 2555240
- DOI: http://doi.org/10.4266/acc.2021.01158
Abstract
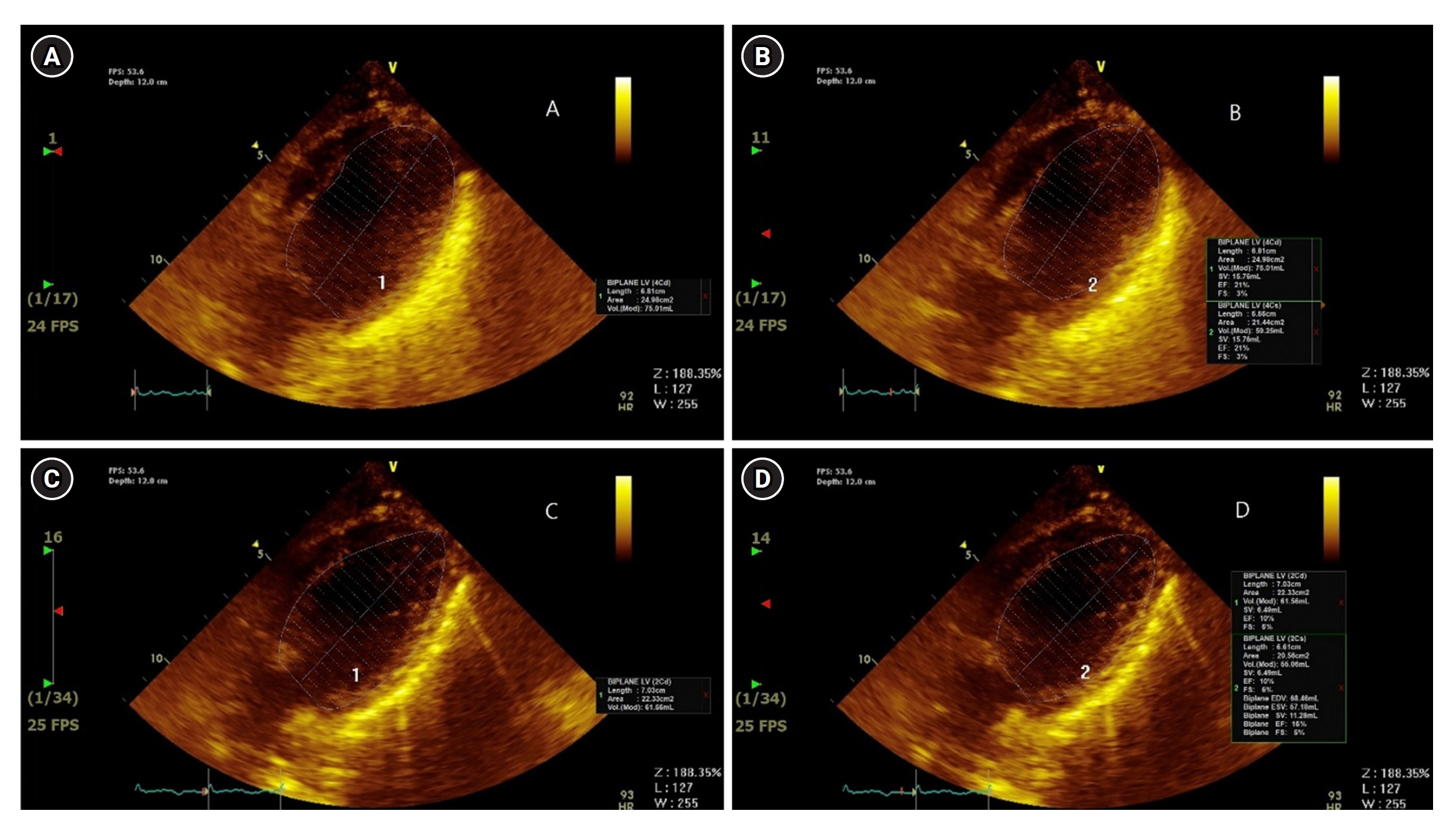
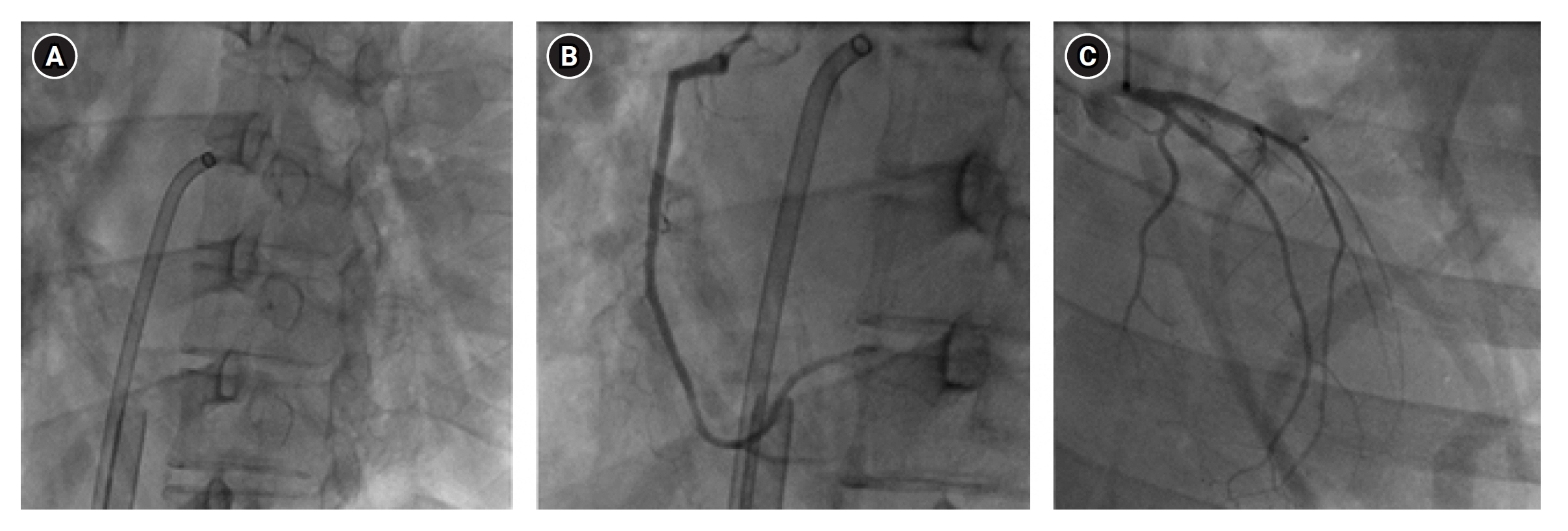
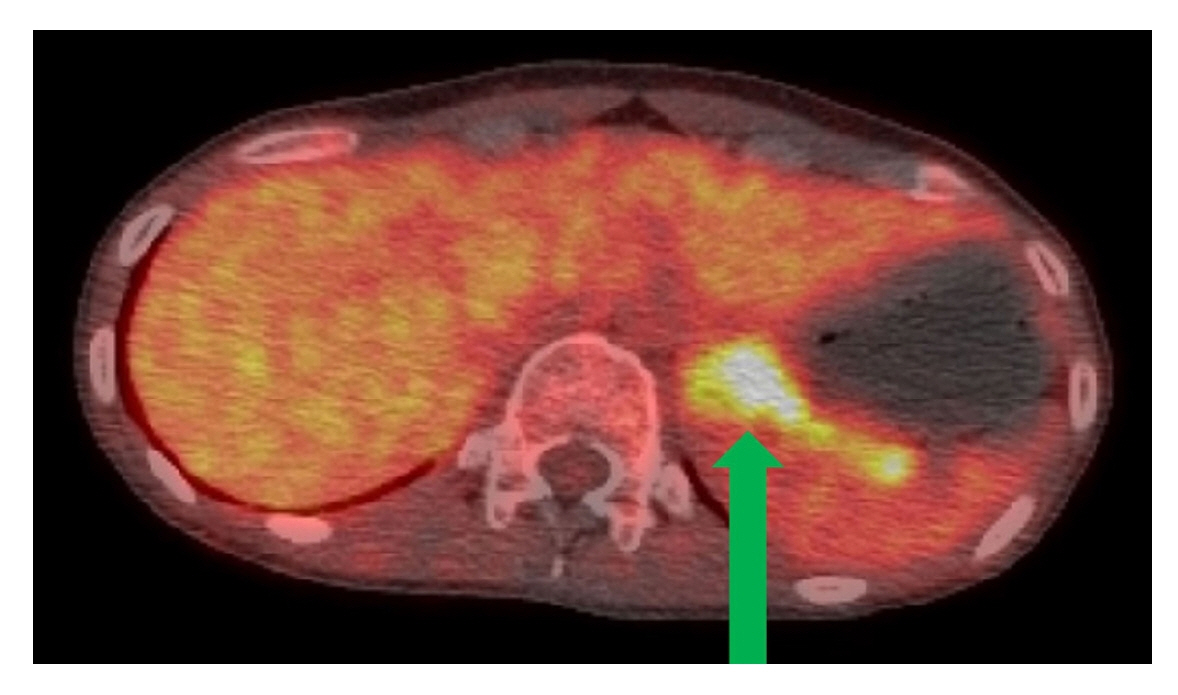
- The main mechanism of Takotsubo cardiomyopathy (TCM) is catecholamine-induced acute myocardial stunning. Pheochromocytoma, a catecholamine-secreting tumor, can cause several cardiovascular complications, including hypertensive crisis, myocardial infarction, toxic myocarditis, and TCM. A 29-year-old woman presented to our hospital with general weakness, vomiting, dyspnea, and chest pain. The patient was nullipara, 28 weeks’ gestation, and had a cachexic morphology. Her cardiac enzyme levels were elevated and bedside echocardiography showed apical akinesia, suggesting TCM. The next day, she could not feel the fetal movement, and an emergency cesarean section was performed. After delivery, the patient experienced cardiac arrest and was transferred to the intensive care unit for cardiopulmonary resuscitation (CPR). Spontaneous circulation returned after 28 minutes of CPR, but cardiogenic shock continued, and extracorporeal membrane oxygenation (ECMO) was initiated. On the third day of ECMO maintenance, left ventricular ejection fraction improved and blood pressure stabilized. On the eighth day after ECMO insertion, it was removed. However, complications of the left leg vessels occurred, and several surgeries and interventions were performed. A left adrenal gland mass was found on computed tomography and was removed while repairing the leg vessels. Pheochromocytoma was diagnosed and left adrenalectomy was performed.
Keyword
Figure
Reference
-
1. Bybee KA, Prasad A. Stress-related cardiomyopathy syndromes. Circulation. 2008; 118:397–409.
Article2. Pelliccia F, Kaski JC, Crea F, Camici PG. Pathophysiology of Takotsubo syndrome. Circulation. 2017; 135:2426–41.
Article3. Nef HM, Möllmann H, Kostin S, Troidl C, Voss S, Weber M, et al. Tako-Tsubo cardiomyopathy: intraindividual structural analysis in the acute phase and after functional recovery. Eur Heart J. 2007; 28:2456–64.
Article4. Galetta F, Franzoni F, Bernini G, Poupak F, Carpi A, Cini G, et al. Cardiovascular complications in patients with pheochromocytoma: a mini-review. Biomed Pharmacother. 2010; 64:505–9.
Article5. Zhang R, Gupta D, Albert SG. Pheochromocytoma as a reversible cause of cardiomyopathy: analysis and review of the literature. Int J Cardiol. 2017; 249:319–23.
Article6. Sood J, Jayaraman L, Kumra VP, Chowbey PK. Laparoscopic approach to pheochromocytoma: is a lower intraabdominal pressure helpful? Anesth Analg. 2006; 102:637–41.
Article7. Santos JR, Brofferio A, Viana B, Pacak K. Catecholamine-induced cardiomyopathy in pheochromocytoma: how to manage a rare complication in a rare disease? Horm Metab Res. 2019; 51:458–69.
Article8. Stolk RF, Bakx C, Mulder J, Timmers HJ, Lenders JW. Is the excess cardiovascular morbidity in pheochromocytoma related to blood pressure or to catecholamines? J Clin Endocrinol Metab. 2013; 98:1100–6.
Article9. Citro R, Giudice R, Mirra M, Petta R, Baldi C, Bossone E, et al. Is Tako-tsubo syndrome in the postpartum period a clinical entity different from peripartum cardiomyopathy? J Cardiovasc Med (Hagerstown). 2013; 14:568–75.
Article10. Oliva R, Angelos P, Kaplan E, Bakris G. Pheochromocytoma in pregnancy: a case series and review. Hypertension. 2010; 55:600–6.11. Agrawal S, Shirani J, Garg L, Singh A, Longo S, Longo A, et al. Pheochromocytoma and stress cardiomyopathy: Insight into pathogenesis. World J Cardiol. 2017; 9:255–60.
Article12. Sircuta C, Lazar A, Azamfirei L, Baranyi M, Vizi ES, Borbély Z. Correlation between the increased release of catecholamines evoked by local anesthetics and their analgesic and adverse effects: role of K(+) channel inhibition. Brain Res Bull. 2016; 124:21–6.13. O'Hara CB, Keyes A, Renwick B, Giel KE, Campbell IC, Schmidt U. Evidence that illness-compatible cues are rewarding in women recovered from anorexia nervosa: a study of the effects of dopamine depletion on eye-blink startle responses. PLoS One. 2016; 11:e0165104.14. Mebazaa A, Seronde MF, Gayat E, Tibazarwa K, Anumba DO, Akrout N, et al. Imbalanced angiogenesis in peripartum cardiomyopathy: diagnostic value of placenta growth factor. Circ J. 2017; 81:1654–61.
Article15. Bauersachs J, König T, van der Meer P, Petrie MC, Hilfiker-Kleiner D, Mbakwem A, et al. Pathophysiology, diagnosis and management of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Study Group on peripartum cardiomyopathy. Eur J Heart Fail. 2019; 21:827–43.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Catastrophic catecholamine-induced cardiomyopathy rescued by extracorporeal membrane oxygenation in recurrent malignant pheochromocytoma
- Catecholamine-Induced Cardiomyopathy associated with Neuroblastoma and Treated with Extracorporeal Membrane Oxygenation as a Bridge to Recovery
- A Case of Pheochromocytoma Presented with Severe Left Ventricular Dysfunction Combined with Cardiogenic Shock after Caesearian Section Delivery Responding to Treatment of Extracorporeal Membrane Oxygenation
- Catastrophic Catecholamine-Induced Cardiomyopathy Mimicking Acute Myocardial Infarction, Rescued by Extracorporeal Membrane Oxygenation (ECMO) in Pheochromocytoma
- Anesthetic experience of pheochromocytoma resection with catecholamine-induced cardiomyopathy and congestive heart failure : A case report