J Korean Neurosurg Soc.
2024 May;67(3):270-279. 10.3340/jkns.2024.0033.
Congenital Intracranial Vascular Malformations in Children : Radiological Overview
- Affiliations
-
- 1Department of Radiology, Seoul National University Hospital, Seoul, Korea
- 2Department of Radiology, Seoul National University College of Medicine, Seoul, Korea
- 3Institute of Radiation Medicine, Seoul National University Medical Research Center, Seoul, Korea
- 4Department of Radiology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2554864
- DOI: http://doi.org/10.3340/jkns.2024.0033
Abstract
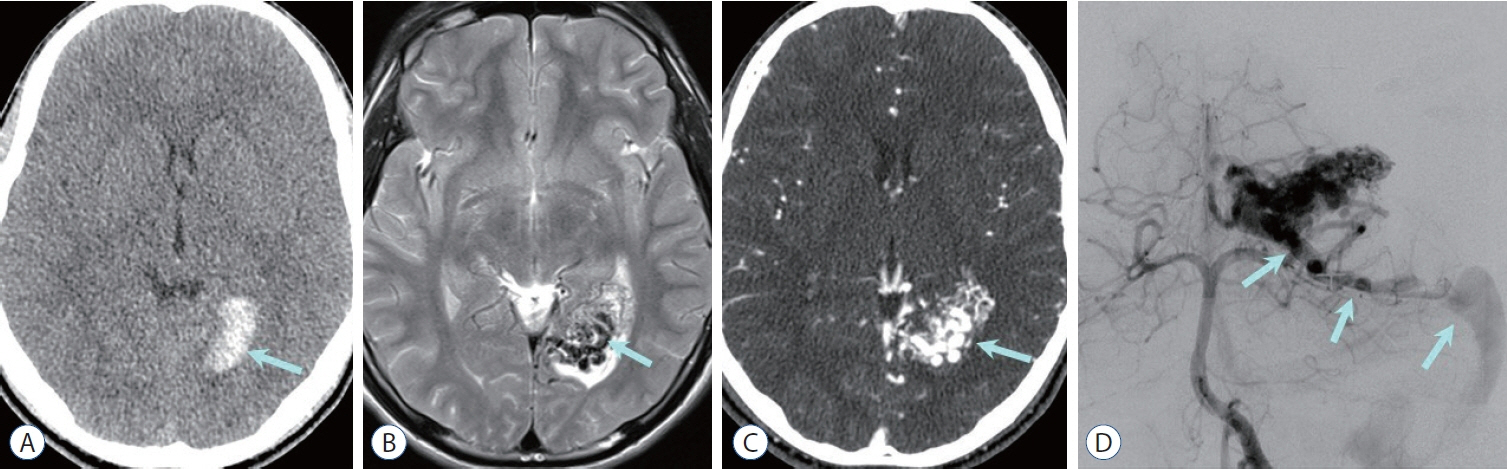
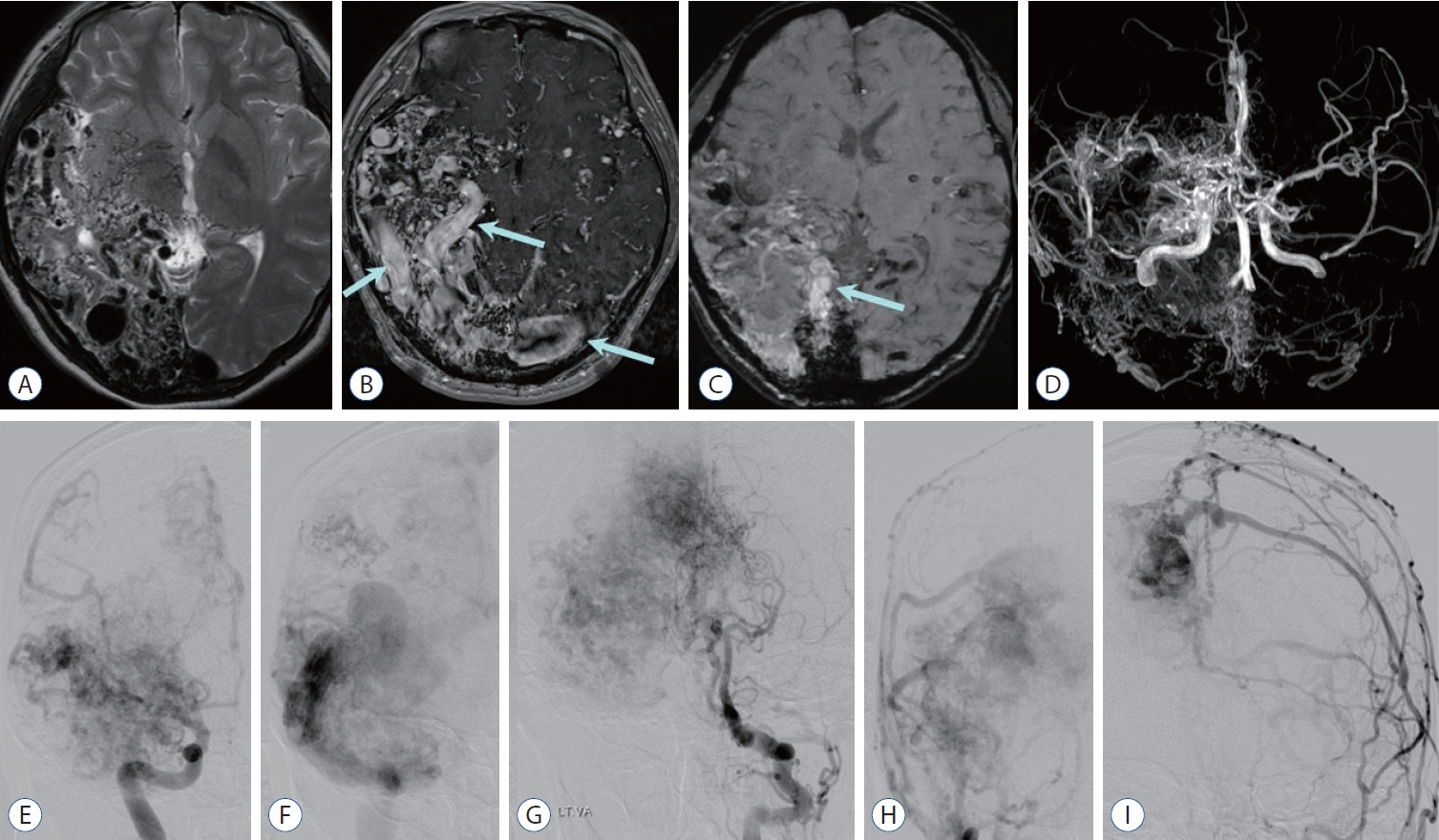
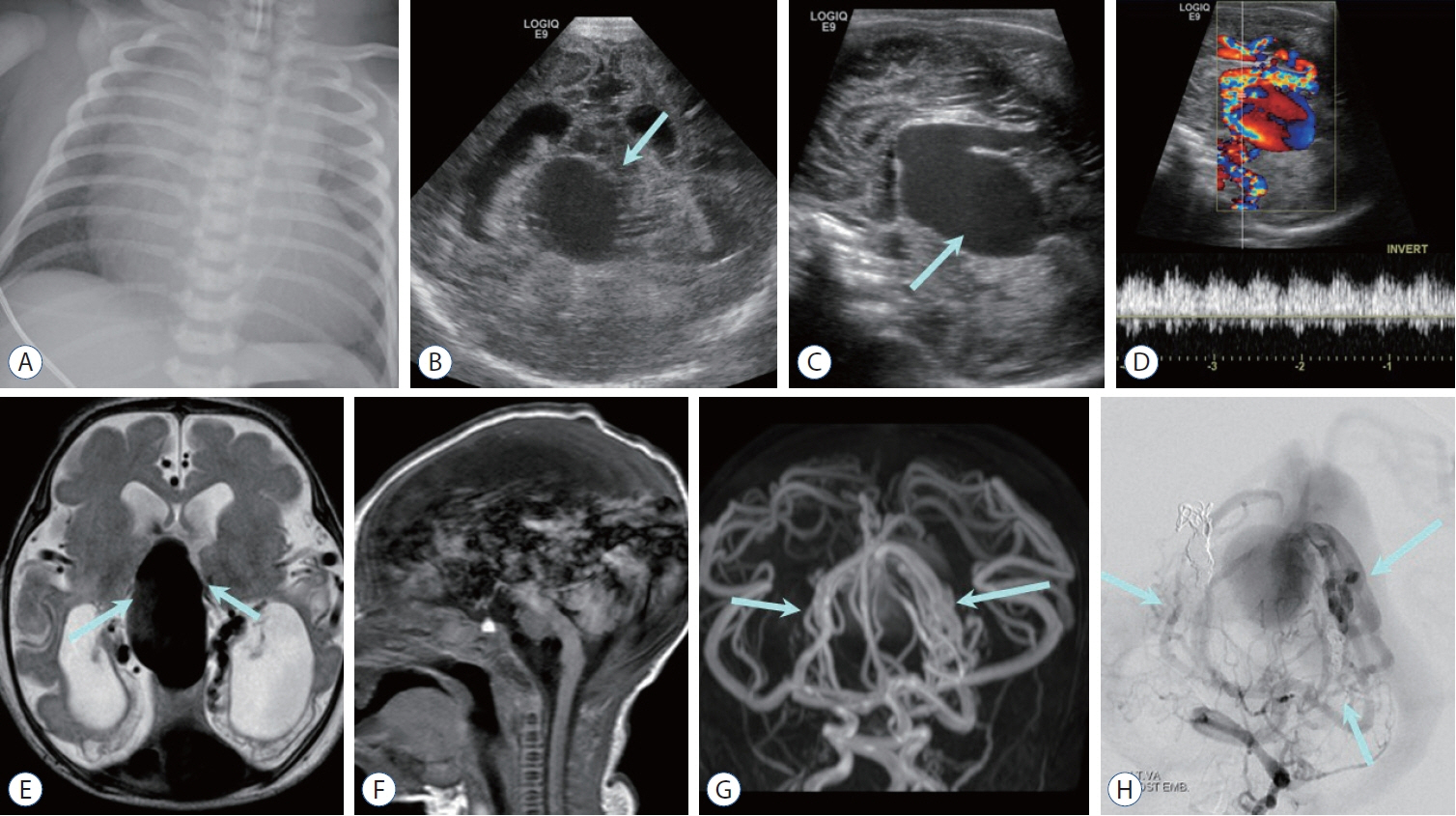
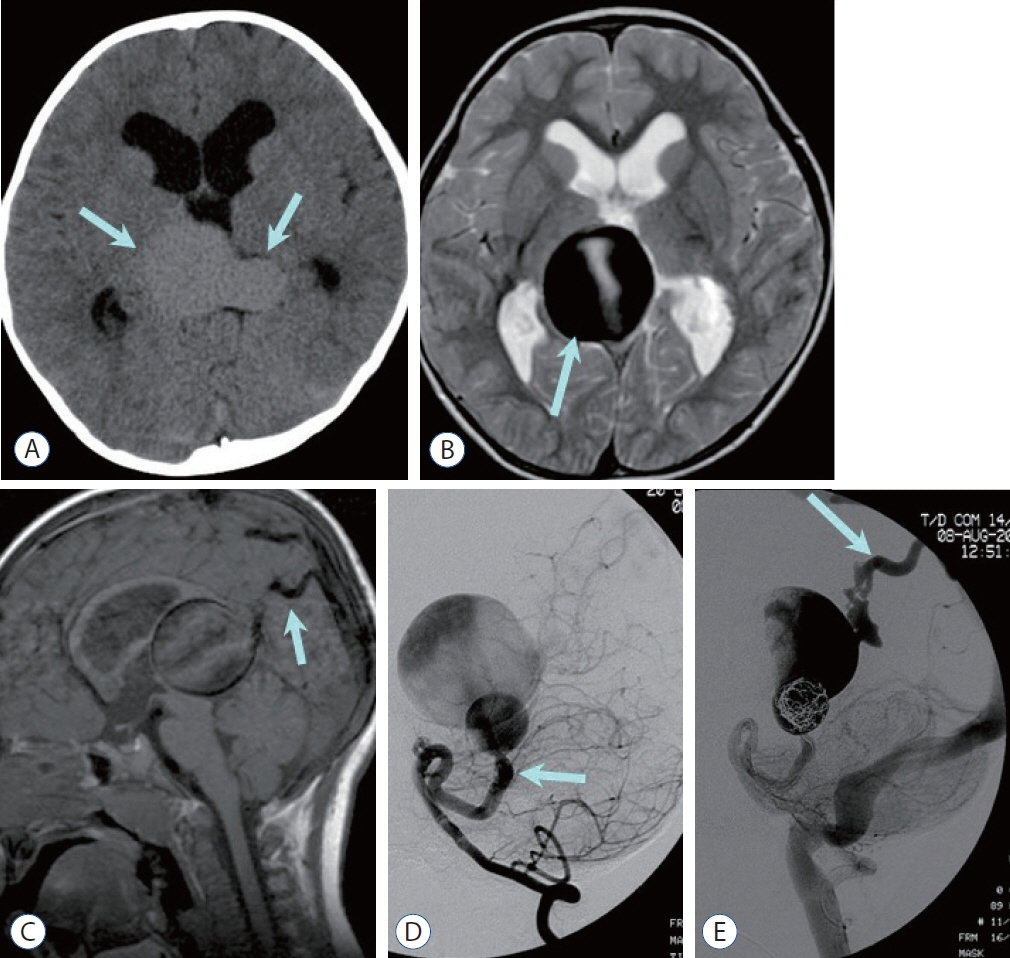
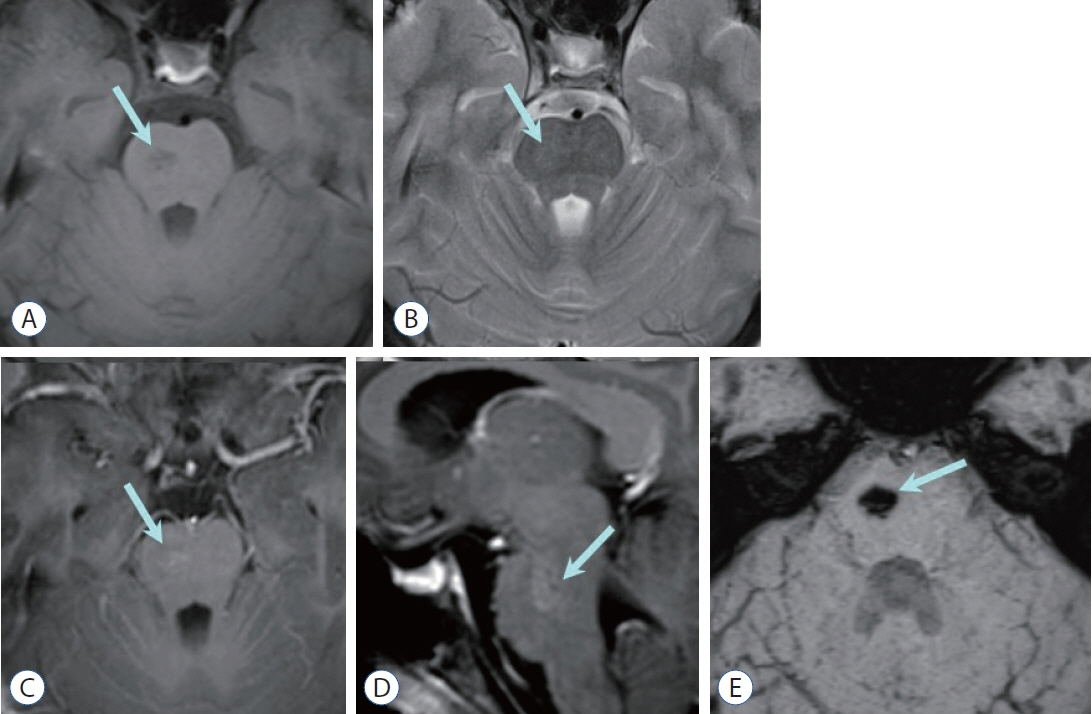
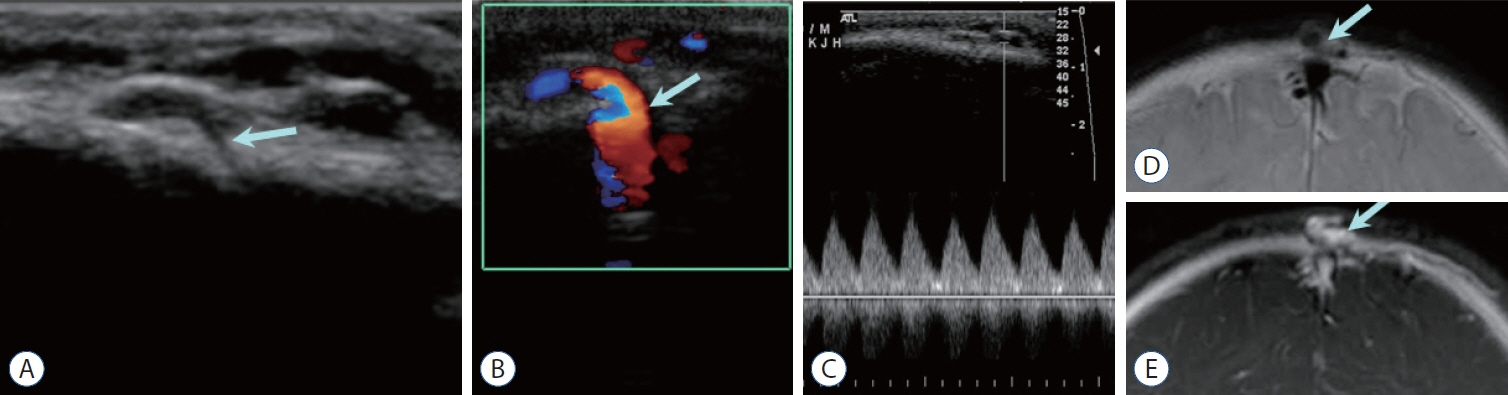
- Prompt medical attention is crucial for congenital intracranial vascular malformations in children and newborns due to potential severe outcomes. Imaging is pivotal for accurate identification, given the diverse risks and treatment strategies. This article aims to enhance the identification and understanding of congenital intracranial vascular abnormalities including arteriovenous malformation, arteriovenous fistula, cavernous malformation, capillary telangiectasia, developmental venous anomaly, and sinus pericranii in pediatric patients.
Keyword
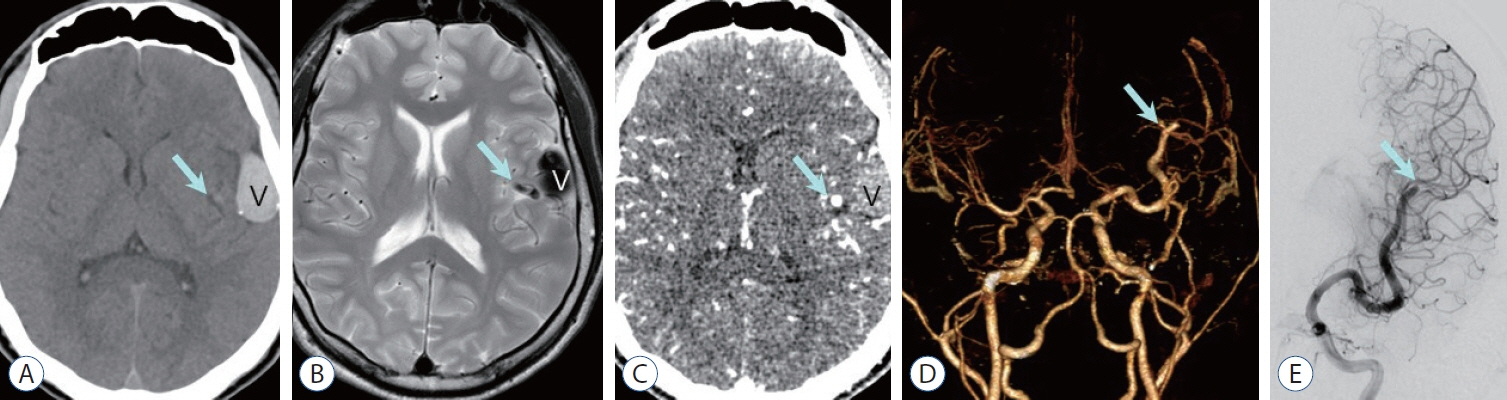
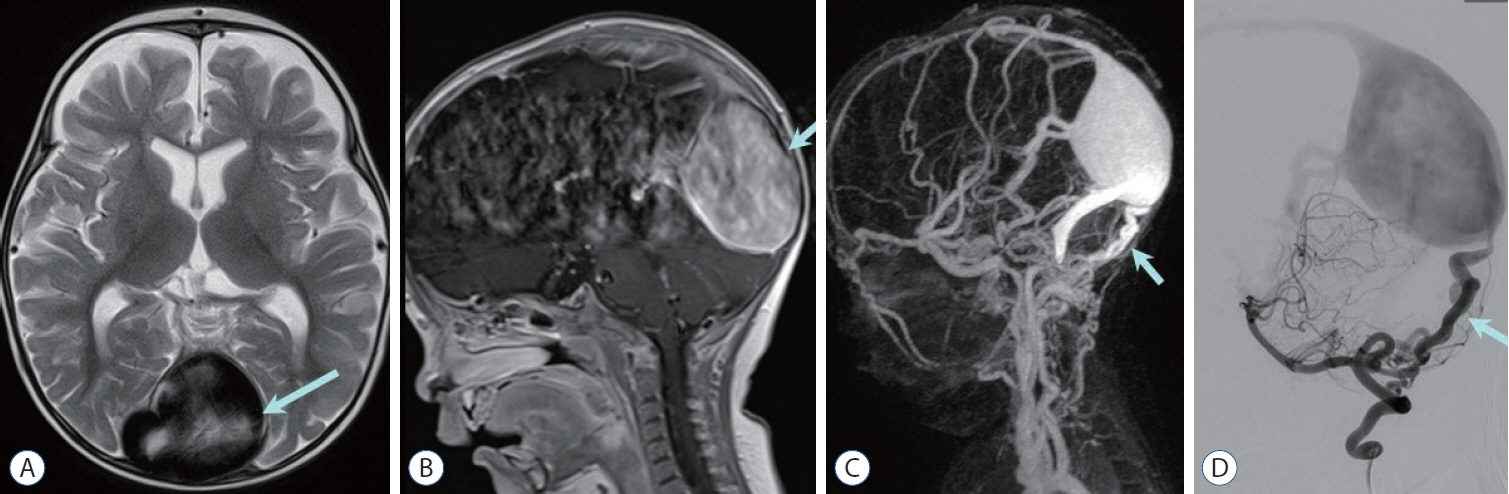
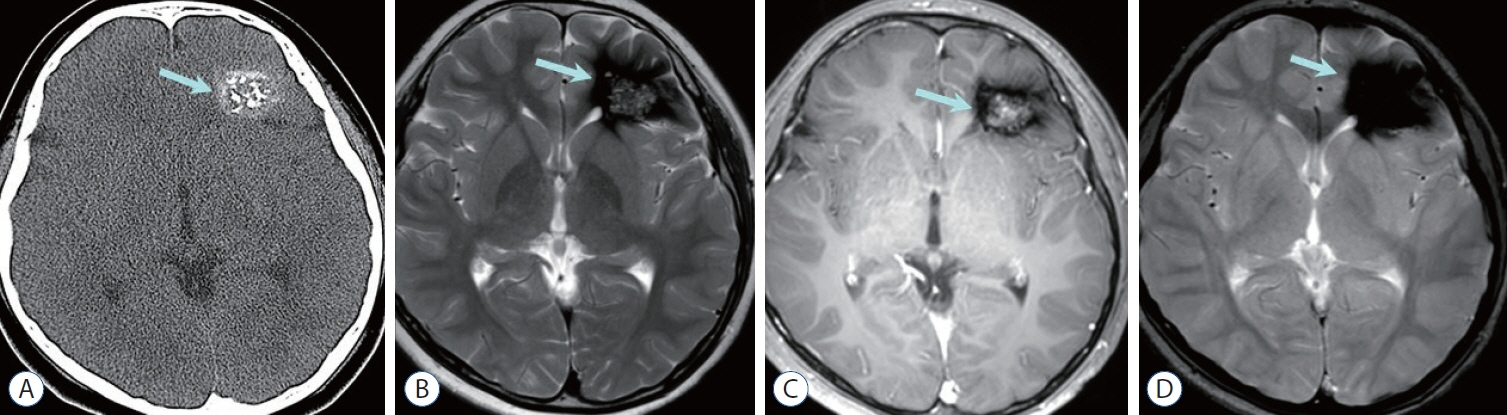
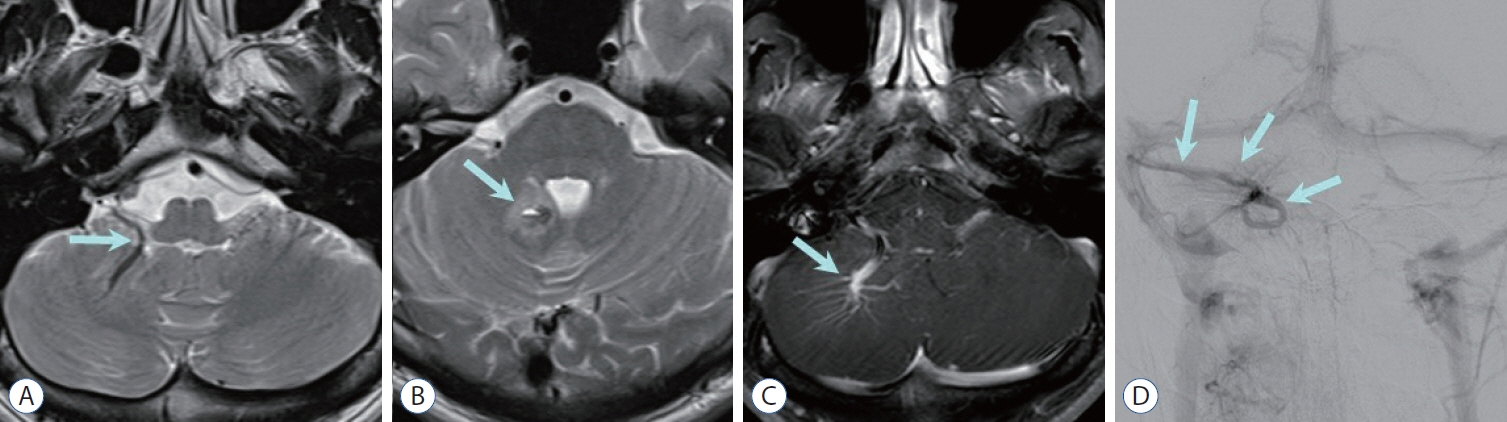
Figure
Reference
-
References
1. Alvarez H, Garcia Monaco R, Rodesch G, Sachet M, Krings T, Lasjaunias P. Vein of galen aneurysmal malformations. Neuroimaging Clin N Am. 17:189–206. 2007.
Article2. Burch EA, Orbach DB. Pediatric central nervous system vascular malformations. Pediatr Radiol. 45 Suppl 3:S463–S472. 2015.
Article3. Chen CJ, Ding D, Derdeyn CP, Lanzino G, Friedlander RM, Southerland AM, et al. Brain arteriovenous malformations: a review of natural history, pathobiology, and interventions. Neurology. 95:917–927. 2020.4. Darsaut TE, Guzman R, Marcellus ML, Edwards MS, Tian L, Do HM, et al. Management of pediatric intracranial arteriovenous malformations: experience with multimodality therapy. Neurosurgery. 69:540–556. discussion 556. 2011.
Article5. Geibprasert S, Pongpech S, Jiarakongmun P, Shroff MM, Armstrong DC, Krings T. Radiologic assessment of brain arteriovenous malformations: what clinicians need to know. Radiographics. 30:483–501. 2010.
Article6. Hacein-Bey L, Konstas AA, Pile-Spellman J. Natural history, current concepts, classification, factors impacting endovascular therapy, and pathophysiology of cerebral and spinal dural arteriovenous fistulas. Clin Neurol Neurosurg. 121:64–75. 2014.
Article7. Hetts SW, Moftakhar P, Maluste N, Fullerton HJ, Cooke DL, Amans MR, et al. Pediatric intracranial dural arteriovenous fistulas: age-related differences in clinical features, angioarchitecture, and treatment outcomes. J Neurosurg Pediatr. 18:602–610. 2016.
Article8. Jaimes C, Machado-Rivas F, Chen K, Bedoya MA, Yang E, Orbach DB. Brain injury in fetuses with vein of Galen malformation and nongalenic arteriovenous fistulas: static snapshot or a portent of more? AJNR Am J Neuroradiol. 43:1036–1041. 2022.
Article9. Krings T, Geibprasert S, Terbrugge K. Classification and endovascular management of pediatric cerebral vascular malformations. Neurosurg Clin N Am. 21:463–482. 2010.
Article10. Liby P, Lomachinsky V, Petrak B, Kyncl M, Charvat F, Padr R, et al. Torcular dural sinus malformations: a single-center case series and a review of literature. Childs Nerv Syst. 36:333–341. 2020.
Article11. LoPresti MA, Ravindra VM, Pyarali M, Goethe E, Gadgil N, Wagner K, et al. Pediatric intracranial arteriovenous malformations: a single-center experience. J Neurosurg Pediatr. 25:151–158. 2019.
Article12. Maleknia PD, Hale AT, Savage C, Blount JP, Rocque BG, Rozzelle CJ, et al. Characteristics and outcomes of pediatric dural arteriovenous fistulas: a systematic review. Childs Nerv Syst. 40:197–204. 2024.
Article13. Mansmann U, Meisel J, Brock M, Rodesch G, Alvarez H, Lasjaunias P. Factors associated with intracranial hemorrhage in cases of cerebral arteriovenous malformation. Neurosurgery. 46:272–279. discussion 279-281. 2000.
Article14. Mokin M, Dumont TM, Levy EI. Novel multimodality imaging techniques for diagnosis and evaluation of arteriovenous malformations. Neurol Clin. 32:225–236. 2014.
Article15. Montaser A, Smith ER. Intracranial vascular abnormalities in children. Pediatr Clin North Am. 68:825–843. 2021.
Article16. Mossa-Basha M, Chen J, Gandhi D. Imaging of cerebral arteriovenous malformations and dural arteriovenous fistulas. Neurosurg Clin N Am. 23:27–42. 2012.
Article17. Paddock M, Lanham S, Gill K, Sinha S, Connolly DJA. Pediatric cerebral cavernous malformations. Pediatr Neurol. 116:74–83. 2021.
Article18. Rai Y, Ogiwara H. Atretic cephalocele associated with sinus pericranii: a single-center analysis. Childs Nerv Syst. 40:543–547. 2024.
Article19. Ravindra VM, Bollo RJ, Eli IM, Griauzde J, Lanpher A, Klein J, et al. A study of pediatric cerebral arteriovenous malformations: clinical presentation, radiological features, and long-term functional and educational outcomes with predictors of sustained neurological deficits. J Neurosurg Pediatr. 24:1–8. 2019.
Article20. Recinos PF, Rahmathulla G, Pearl M, Recinos VR, Jallo GI, Gailloud P, et al. Vein of Galen malformations: epidemiology, clinical presentations, management. Neurosurg Clin N Am. 23:165–177. 2012.
Article21. Requejo F, Teplisky D, Dutra MLG, Mouratian DM, Kikano R, Nguyen TN, et al. Pediatric interventional neuroradiology. Semin Neurol. 43:408–418. 2023.
Article22. Ruíz DS, Yilmaz H, Gailloud P. Cerebral developmental venous anomalies: current concepts. Ann Neurol. 66:271–283. 2009.23. Sabayan B, Lineback C, Viswanathan A, Leslie-Mazwi TM, Shaibani A. Central nervous system vascular malformations: a clinical review. Ann Clin Transl Neurol. 8:504–522. 2021.24. Santos AN, Rauschenbach L, Saban D, Chen B, Herten A, Dinger TF, et al. Natural course of cerebral cavernous malformations in children: a five-year follow-up study. Stroke. 53:817–824. 2022.25. Sarma A, Martin D, Pruthi S, Jones R, Little SB. Imaging the cerebral veins in pediatric patients: beyond dural venous sinus thrombosis. Radiographics. 43:e220129. 2023.26. Shtaya A, Millar J, Sparrow O. Multimodality management and outcomes of brain arterio-venous malformations (AVMs) in children: personal experience and review of the literature, with specific emphasis on age at first AVM bleed. Childs Nerv Syst. 33:573–581. 2017.27. Zuniega RRA, Santos JA, Galsim RJG, Elevazo JS. Neonatal giant dural sinus ectasia: a multimodality imaging approach. BMJ Case Rep. 14:e242439. 2021.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pediatric Cerebral Vascular Malformations : Current and Future Perspectives
- 3 Cases of Surgically Treated Arteriovenous Malformations of the Brain
- Imaging of Intracranial Hemorrhage
- Spontaneous Intracranial Hemorrhage in Children: Analysis of Clinical Characteristics
- A Review of the Current State and Future Directions for Management of Scalp and Facial Vascular Malformations