J Yeungnam Med Sci.
2024 Apr;41(2):113-119. 10.12701/jyms.2024.00024.
Performance of the BD MAX MDR-TB assay in a clinical setting and its impact on the clinical course of patients with pulmonary tuberculosis: a retrospective before-after study
- Affiliations
-
- 1Department of Internal Medicine, Wonkwang University Sanbon Hospital, Gunpo, Korea
- 2Department of Laboratory Medicine, Wonkwang University Sanbon Hospital, Gunpo, Korea
- KMID: 2554772
- DOI: http://doi.org/10.12701/jyms.2024.00024
Abstract
- Background
Missing isoniazid (INH) resistance during tuberculosis (TB) diagnosis can worsen the outcomes of INH-resistant TB. The BD MAX MDR-TB assay (BD MAX) facilitates the rapid detection of TB and INH and rifampin (RIF) resistance; however, data related to its performance in clinical setting remain limited. Moreover, its effect on treatment outcomes has not yet been studied.
Methods
We compared the performance of BD MAX for the detection of INH/RIF resistances to that of the line probe assay (LPA) in patients with pulmonary TB (PTB), using the results of a phenotypic drug sensitivity test as a reference standard. The treatment outcomes of patients who used BD MAX were compared with those of patients who did not.
Results
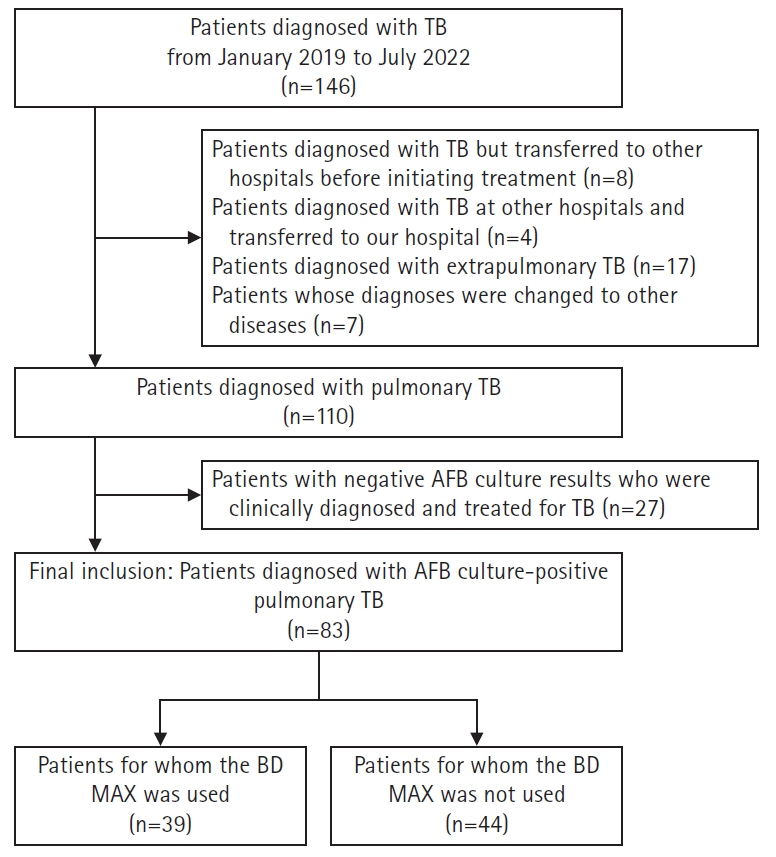
Of the 83 patients included in the study, the BD MAX was used for an initial PTB diagnosis in 39 patients. The sensitivity of BD MAX for detecting PTB was 79.5%. The sensitivity and specificity of BD MAX for INH resistance were both 100%, whereas these were 50.0% and 95.8%, respectively, for RIF resistance. The sensitivity and specificity of BD MAX were comparable to those of LPA. The BD MAX group had a shorter time interval from specimen request to the initiation of anti-TB drugs (2.0 days vs. 5.5 days, p=0.001).
Conclusion
BD MAX showed comparable performance to conventional tests for detecting PTB and INH/RIF resistances. The implementation of BD MAX as a diagnostic tool for PTB resulted in a shorter turnaround time for the initiation of PTB treatment.
Figure
Reference
-
References
1. World Health Organization (WHO). Global tuberculosis report 2023 [Internet]. Geneva, Switzerland: WHO;2023. [cited 2024 Jan 4]. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2023.2. Korea Disease Control and Prevention Agency (KDCA). Annual report on the notified tuberculosis in Korea, 2022 [Internet]. Cheongju, Korea: KDCA;2023. [cited 2024 Jan 4]. https://www.kdca.go.kr/board/board.es?mid=a31001000000&bid=0130&act=view&list_no=723921&tag=&nPage=1.3. Jhun BW, Koh WJ. Treatment of isoniazid-resistant pulmonary tuberculosis. Tuberc Respir Dis (Seoul). 2020; 83:20–30.
Article4. Jhun BW, Huh HJ, Koh WJ. Diagnosis of pulmonary tuberculosis. J Korean Med Assoc. 2019; 62:18–24.
Article5. Kim YW, Seong MW, Kim TS, Yoo CG, Kim YW, Han SK, et al. Evaluation of Xpert(®) MTB/RIF assay: diagnosis and treatment outcomes in rifampicin-resistant tuberculosis. Int J Tuberc Lung Dis. 2015; 19:1216–21.
Article6. World Health Organization (WHO). WHO consolidated guidelines on tuberculosis: module 3: diagnosis–rapid diagnostics for tuberculosis detection [Internet]. Geneva, Switzerland: WHO;2020. [cited 2024 Jan 4]. https://www.who.int/publications/i/item/9789240029415.7. Miotto P, Tessema B, Tagliani E, Chindelevitch L, Starks AM, Emerson C, et al. A standardised method for interpreting the association between mutations and phenotypic drug resistance in Mycobacterium tuberculosis. Eur Respir J. 2017; 50:1701354.8. Yadav RN, Kumar Singh B, Sharma R, Chaubey J, Sinha S, Jorwal P. Comparative performance of line probe assay (version 2) and Xpert MTB/RIF assay for early diagnosis of rifampicin-resistant pulmonary tuberculosis. Tuberc Respir Dis (Seoul). 2021; 84:237–44.9. Nathavitharana RR, Cudahy PG, Schumacher SG, Steingart KR, Pai M, Denkinger CM. Accuracy of line probe assays for the diagnosis of pulmonary and multidrug-resistant tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2017; 49:1601075.
Article10. World Health Organization (WHO). The use of molecular line probe assay for the detection of resistance to isoniazid and rifampicin: policy update [Internet]. Geneva, Switzerland: WHO;2016. [cited 2024 Jan 4]. https://apps.who.int/iris/handle/10665/250586.11. World Health Organization (WHO). WHO consolidated guidelines on tuberculosis: module 4: treatment: drug-resistant tuberculosis treatment [Internet]. Geneva, Switzerland: WHO;2020. [cited 2024 Jan 4]. https://www.who.int/publications/i/item/9789240007048.12. Menzies D, Benedetti A, Paydar A, Royce S, Madhukar P, Burman W, et al. Standardized treatment of active tuberculosis in patients with previous treatment and/or with mono-resistance to isoniazid: a systematic review and meta-analysis. PLoS Med. 2009; 6:e1000150.
Article13. Gegia M, Winters N, Benedetti A, van Soolingen D, Menzies D. Treatment of isoniazid-resistant tuberculosis with first-line drugs: a systematic review and meta-analysis. Lancet Infect Dis. 2017; 17:223–34.
Article14. Romanowski K, Campbell JR, Oxlade O, Fregonese F, Menzies D, Johnston JC. The impact of improved detection and treatment of isoniazid resistant tuberculosis on prevalence of multi-drug resistant tuberculosis: a modelling study. PLoS One. 2019; 14:e0211355.
Article15. Shah M, Paradis S, Betz J, Beylis N, Bharadwaj R, Caceres T, et al. Multicenter study of the accuracy of the BD MAX multidrug-resistant tuberculosis assay for detection of mycobacterium tuberculosis complex and mutations associated with resistance to rifampin and isoniazid. Clin Infect Dis. 2020; 71:1161–7.
Article16. Beutler M, Plesnik S, Mihalic M, Olbrich L, Heinrich N, Schumacher S, et al. A pre-clinical validation plan to evaluate analytical sensitivities of molecular diagnostics such as BD MAX MDR-TB, Xpert MTB/Rif Ultra and FluoroType MTB. PLoS One. 2020; 15:e0227215.
Article17. Ciesielczuk H, Kouvas N, North N, Buchanan R, Tiberi S. Evaluation of the BD MAX™ MDR-TB assay in a real-world setting for the diagnosis of pulmonary and extra-pulmonary TB. Eur J Clin Microbiol Infect Dis. 2020; 39:1321–7.
Article18. Hofmann-Thiel S, Plesnik S, Mihalic M, Heiß-Neumann M, Avsar K, Beutler M, et al. Clinical evaluation of BD MAX MDR-TB assay for direct detection of mycobacterium tuberculosis complex and resistance markers. J Mol Diagn. 2020; 22:1280–6.
Article19. Linh NN, Viney K, Gegia M, Falzon D, Glaziou P, Floyd K, et al. World Health Organization treatment outcome definitions for tuberculosis: 2021 update. Eur Respir J. 2021; 58:2100804.
Article20. Ko Y, Lee HK, Lee YS, Kim MY, Shin JH, Shim EJ, et al. Accuracy of Xpert(®) MTB/RIF assay compared with AdvanSure™ TB/NTM real-time PCR using bronchoscopy specimens. Int J Tuberc Lung Dis. 2016; 20:115–20.
Article21. Son E, Jang J, Kim T, Jang JH, Chung JH, Seol HY, et al. Head-to-head comparison between Xpert MTB/RIF assay and real-time polymerase chain reaction assay using bronchial washing specimens for tuberculosis diagnosis. Tuberc Respir Dis (Seoul). 2022; 85:89–95.
Article22. Lee HY, Seong MW, Park SS, Hwang SS, Lee J, Park YS, et al. Diagnostic accuracy of Xpert® MTB/RIF on bronchoscopy specimens in patients with suspected pulmonary tuberculosis. Int J Tuberc Lung Dis. 2013; 17:917–21.
Article23. Jo YS, Park JH, Lee JK, Heo EY, Chung HS, Kim DK. Discordance between MTB/RIF and real-time tuberculosis-specific polymerase chain reaction assay in bronchial washing specimen and its clinical implications. PLoS One. 2016; 11:e0164923.
Article24. Kwak SH, Choi JS, Lee EH, Lee SH, Leem AY, Lee SH, et al. Characteristics and risk factors associated with missed diagnosis in patients with smear-negative pulmonary tuberculosis. Korean J Intern Med. 2021; 36(Suppl 1):S151–9.
Article25. Kwak N, Choi SM, Lee J, Park YS, Lee CH, Lee SM, et al. Diagnostic accuracy and turnaround time of the Xpert MTB/RIF assay in routine clinical practice. PLoS One. 2013; 8:e77456.
Article26. Kim J, Kwak N, Lee HY, Kim TS, Kim CK, Han SK, et al. Effect of drug resistance on negative conversion of sputum culture in patients with pulmonary tuberculosis. Int J Infect Dis. 2016; 42:64–8.
Article27. Singh BK, Sharma R, Kodan P, Soneja M, Jorwal P, Nischal N, et al. Diagnostic evaluation of non-interpretable results associated with rpoB gene in genotype MTBDRplus ver 2.0. Tuberc Respir Dis (Seoul). 2020; 83:289–94.
Article28. Rufai SB, Kumar P, Singh A, Prajapati S, Balooni V, Singh S. Comparison of Xpert MTB/RIF with line probe assay for detection of rifampin-monoresistant Mycobacterium tuberculosis. J Clin Microbiol. 2014; 52:1846–52.
Article29. Hughes D, Brandis G. Rifampicin resistance: fitness costs and the significance of compensatory evolution. Antibiotics (Basel). 2013; 2:206–16.
Article30. Zetola NM, Shin SS, Tumedi KA, Moeti K, Ncube R, Nicol M, et al. Mixed Mycobacterium tuberculosis complex infections and false-negative results for rifampin resistance by GeneXpert MTB/RIF are associated with poor clinical outcomes. J Clin Microbiol. 2014; 52:2422–9.
Article31. Georghiou SB, Schumacher SG, Rodwell TC, Colman RE, Miotto P, Gilpin C, et al. Guidance for studies evaluating the accuracy of rapid tuberculosis drug-susceptibility tests. J Infect Dis. 2019; 220(Suppl 3):S126–35.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnostic Accuracy of BD MAX MDR-TB Assay Performed on Bronchoscopy Specimens in Patients with Suspected Pulmonary Tuberculosis
- Current status of drug-resistant tuberculosis and its treatment
- Diagnosis and treatment of multidrug-resistant tuberculosis
- Multidrug-resistant Tuberculosis
- Concurrent, Prolonged Use of Bedaquiline and Delamanid for Multidrug-Resistant Tuberculosis