Endocrinol Metab.
2024 Apr;39(2):255-261. 10.3803/EnM.2023.1908.
Acquired Forms of Fibroblast Growth Factor 23-Related Hypophosphatemic Osteomalacia
- Affiliations
-
- 1Division of Nephrology and Endocrinology, The University of Tokyo Hospital, Tokyo, Japan
- 2Osteoporosis Center, The University of Tokyo Hospital, Tokyo, Japan
- KMID: 2554629
- DOI: http://doi.org/10.3803/EnM.2023.1908
Abstract
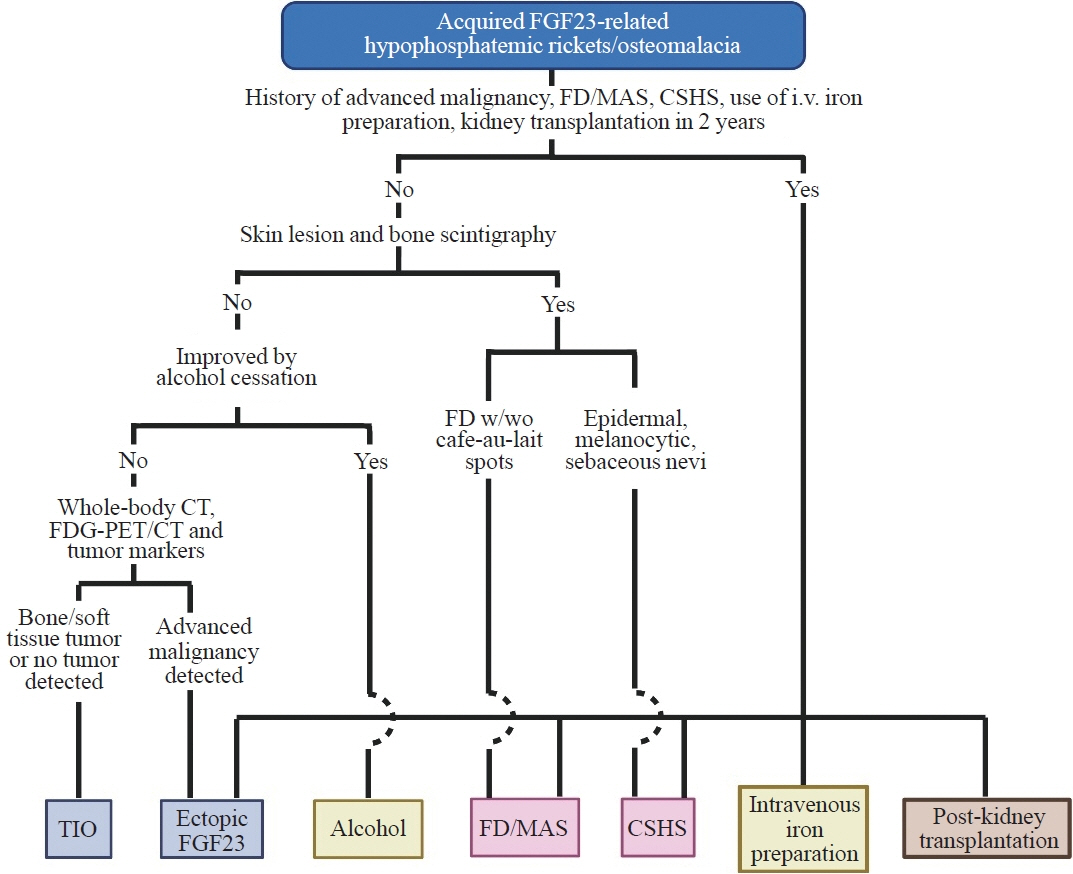
- Fibroblast growth factor 23 (FGF23) is a pivotal humoral factor for the regulation of serum phosphate levels and was first identified in patients with autosomal dominant hypophosphatemic rickets and tumor-induced osteomalacia (TIO), the most common form of acquired FGF23-related hypophosphatemic rickets/osteomalacia (FGF23rHR). After the identification of FGF23, many other inherited and acquired forms of FGF23rHR were reported. In this review article, the detailed features of each acquired FGF23rHR are discussed, including TIO, ectopic FGF23 syndrome with malignancy, fibrous dysplasia/McCune-Albright syndrome, Schimmelpenning-Feuerstein-Mims syndrome/cutaneous skeletal hypophosphatemia syndrome, intravenous iron preparation-induced FGF23rHR, alcohol consumption-induced FGF23rHR, and post-kidney transplantation hypophosphatemia. Then, an approach for the differential diagnosis and therapeutic options for each disorder are concisely introduced. Currently, the majority of endocrinologists might only consider TIO when encountering patients with acquired FGF23rHR; an adequate differential diagnosis can reduce medical costs and invasive procedures such as positron emission tomography/computed tomography and venous sampling to identify FGF23-producing tumors. Furthermore, some acquired FGF23rHRs, such as intravenous iron preparation/alcohol consumption-induced FGF23rHR, require only cessation of drugs or alcohol to achieve full recovery from osteomalacia.
Keyword
Figure
Reference
-
1. Ito N, Hidaka N, Kato H. The pathophysiology of hypophosphatemia. Best Pract Res Clin Endocrinol Metab. 2023; 101851.
Article2. Takashi Y, Kosako H, Sawatsubashi S, Kinoshita Y, Ito N, Tsoumpra MK, et al. Activation of unliganded FGF receptor by extracellular phosphate potentiates proteolytic protection of FGF23 by its O-glycosylation. Proc Natl Acad Sci U S A. 2019; 116:11418–27.
Article3. Fukumoto S. FGF23-related hypophosphatemic rickets/osteomalacia: diagnosis and new treatment. J Mol Endocrinol. 2021; 66:R57–65.
Article4. Ito N, Fukumoto S. Congenital hyperphosphatemic conditions caused by the deficient activity of FGF23. Calcif Tissue Int. 2021; 108:104–15.
Article5. ADHR Consortium. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet. 2000; 26:345–8.6. Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci U S A. 2001; 98:6500–5.
Article7. Lee JC, Jeng YM, Su SY, Wu CT, Tsai KS, Lee CH, et al. Identification of a novel FN1-FGFR1 genetic fusion as a frequent event in phosphaturic mesenchymal tumour. J Pathol. 2015; 235:539–45.8. Lee JC, Su SY, Changou CA, Yang RS, Tsai KS, Collins MT, et al. Characterization of FN1-FGFR1 and novel FN1-FGF1 fusion genes in a large series of phosphaturic mesenchymal tumors. Mod Pathol. 2016; 29:1335–46.
Article9. Kinoshita Y, Takashi Y, Ito N, Ikegawa S, Mano H, Ushiku T, et al. Ectopic expression of Klotho in fibroblast growth factor 23 (FGF23)-producing tumors that cause tumor-induced rickets/osteomalacia (TIO). Bone Rep. 2018; 10:100192.
Article10. Minisola S, Peacock M, Fukumoto S, Cipriani C, Pepe J, Tella SH, et al. Tumour-induced osteomalacia. Nat Rev Dis Primers. 2017; 3:17044.
Article11. Folpe AL, Fanburg-Smith JC, Billings SD, Bisceglia M, Bertoni F, Cho JY, et al. Most osteomalacia-associated mesenchymal tumors are a single histopathologic entity: an analysis of 32 cases and a comprehensive review of the literature. Am J Surg Pathol. 2004; 28:1–30.
Article12. El-Maouche D, Sadowski SM, Papadakis GZ, Guthrie L, Cottle-Delisle C, Merkel R, et al. 68Ga-DOTATATE for tumor localization in tumor-induced osteomalacia. J Clin Endocrinol Metab. 2016; 101:3575–81.13. Kato H, Koga M, Kinoshita Y, Hidaka N, Hoshino Y, Takashi Y, et al. Utility of multimodality approach including systemic FGF23 venous sampling in localizing phosphaturic mesenchymal tumors. J Endocr Soc. 2022; 7:bvac181.
Article14. Hidaka N, Koga M, Kimura S, Hoshino Y, Kato H, Kinoshita Y, et al. Clinical challenges in diagnosis, tumor localization and treatment of tumor-induced osteomalacia: outcome of a retrospective surveillance. J Bone Miner Res. 2022; 37:1479–88.
Article15. Imanishi Y, Ito N, Rhee Y, Takeuchi Y, Shin CS, Takahashi Y, et al. Interim analysis of a phase 2 open-label trial assessing burosumab efficacy and safety in patients with tumor-induced osteomalacia. J Bone Miner Res. 2021; 36:262–70.
Article16. Jan de Beur SM, Miller PD, Weber TJ, Peacock M, Insogna K, Kumar R, et al. Burosumab for the treatment of tumorinduced osteomalacia. J Bone Miner Res. 2021; 36:627–35.
Article17. Hartley IR, Miller CB, Papadakis GZ, Bergwitz C, Del Rivero J, Blau JE, et al. Targeted FGFR blockade for the treatment of tumor-induced osteomalacia. N Engl J Med. 2020; 383:1387–9.
Article18. Kato H, Kimura S, Taguchi M, Sunouchi T, Hoshino Y, Hidaka N, et al. FGF23-related hypophosphatemia in a patient with small cell lung cancer: a case report and literature review. Endocr J. 2023; 70:1005–13.
Article19. Leaf DE, Pereira RC, Bazari H, Juppner H. Oncogenic osteomalacia due to FGF23-expressing colon adenocarcinoma. J Clin Endocrinol Metab. 2013; 98:887–91.
Article20. Sauder A, Wiernek S, Dai X, Pereira R, Yudd M, Patel C, et al. FGF23-associated tumor-induced osteomalacia in a patient with small cell carcinoma: a case report and regulatory mechanism study. Int J Surg Pathol. 2016; 24:116–20.
Article21. Riminucci M, Collins MT, Fedarko NS, Cherman N, Corsi A, White KE, et al. FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest. 2003; 112:683–92.
Article22. Gun ZH, Osamor C 3rd, Taylor J, Li X, Szymczuk V, Boyce AM. Serum phosphorus as a driver of skeletal morbidity in fibrous dysplasia. J Clin Endocrinol Metab. 2024; 109:1334–40.
Article23. Gladding A, Szymczuk V, Auble BA, Boyce AM. Burosumab treatment for fibrous dysplasia. Bone. 2021; 150:116004.
Article24. Avitan-Hersh E, Tatur S, Indelman M, Gepstein V, Shreter R, Hershkovitz D, et al. Postzygotic HRAS mutation causing both keratinocytic epidermal nevus and thymoma and associated with bone dysplasia and hypophosphatemia due to elevated FGF23. J Clin Endocrinol Metab. 2014; 99:E132–6.
Article25. Lim YH, Ovejero D, Sugarman JS, Deklotz CM, Maruri A, Eichenfield LF, et al. Multilineage somatic activating mutations in HRAS and NRAS cause mosaic cutaneous and skeletal lesions, elevated FGF23 and hypophosphatemia. Hum Mol Genet. 2014; 23:397–407.
Article26. Huynh C, Gillis A, Fazendin J, Abdullatif H. A case report to assess the safety and efficacy of burosumab, an investigational antibody to FGF23, in a single pediatric patient with epidermal nevus syndrome and associated hypophosphatemic rickets. Bone Rep. 2022; 17:101605.
Article27. Sugarman J, Maruri A, Hamilton DJ, Tabatabai L, Luca D, Cimms T, et al. The efficacy and safety of burosumab in two patients with cutaneous skeletal hypophosphatemia syndrome. Bone. 2023; 166:116598.
Article28. Shimizu Y, Tada Y, Yamauchi M, Okamoto T, Suzuki H, Ito N, et al. Hypophosphatemia induced by intravenous administration of saccharated ferric oxide: another form of FGF23-related hypophosphatemia. Bone. 2009; 45:814–6.
Article29. Schouten BJ, Doogue MP, Soule SG, Hunt PJ. Iron polymaltose-induced FGF23 elevation complicated by hypophosphataemic osteomalacia. Ann Clin Biochem. 2009; 46(Pt 2):167–9.
Article30. Wolf M, Koch TA, Bregman DB. Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J Bone Miner Res. 2013; 28:1793–803.
Article31. Wolf M, Chertow GM, Macdougall IC, Kaper R, Krop J, Strauss W. Randomized trial of intravenous iron-induced hypophosphatemia. JCI Insight. 2018; 3:e124486.
Article32. Wolf M. Update on fibroblast growth factor 23 in chronic kidney disease. Kidney Int. 2012; 82:737–47.
Article33. Schaefer B, Zoller H, Wolf M. Risk factors for and effects of persistent and severe hypophosphatemia following ferric carboxymaltose. J Clin Endocrinol Metab. 2022; 107:1009–19.
Article34. Wolf M, Rubin J, Achebe M, Econs MJ, Peacock M, Imel EA, et al. Effects of iron isomaltoside vs ferric carboxymaltose on hypophosphatemia in iron-deficiency anemia: two randomized clinical trials. JAMA. 2020; 323:432–43.
Article35. Hidaka N, Kato H, Koga M, Katsura M, Oyama Y, Kinoshita Y, et al. Induction of FGF23-related hypophosphatemic osteomalacia by alcohol consumption. Bone Rep. 2021; 15:101144.
Article36. Bhan I, Shah A, Holmes J, Isakova T, Gutierrez O, Burnett SM, et al. Post-transplant hypophosphatemia: tertiary ‘Hyper-phosphatoninism’? Kidney Int. 2006; 70:1486–94.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ga68-DOTA Peptide PET/CT to Detect Occult Mesenchymal Tumor-Inducing Osteomalacia: A Case Series of Three Patients
- A FGF23-Positive Maxillary Sinus Tumor Associated with Oncogenic Osteomalacia
- Diagnostic Modalities for FGF23-Producing Tumors in Patients with Tumor-Induced Osteomalacia
- Skeletal mineralization: mechanisms and diseases
- A Case of Sporadic Nonfamilial Hypophosphatemic Osteomalacia