Restor Dent Endod.
2024 Feb;49(1):e6. 10.5395/rde.2024.49.e6.
Effect of different storage media on elemental analysis and microhardness of cervical cavity margins restored with a bioactive material
- Affiliations
-
- 1Conservative Dentistry Department, Faculty of Dentistry, Mansoura University, Mansoura, Egypt
- 2Department of Bioscience Research, College of Dentistry, University of Tennessee Health Science Center, Memphis, TN, USA
- 3The Forsyth Institute, Cambridge, MA, USA
- KMID: 2554134
- DOI: http://doi.org/10.5395/rde.2024.49.e6
Abstract
Objectives
This study aimed to investigate the elemental analysis and microhardness of a bioactive material (Activa) and marginal tooth structure after storage in different media.
Materials and Methods
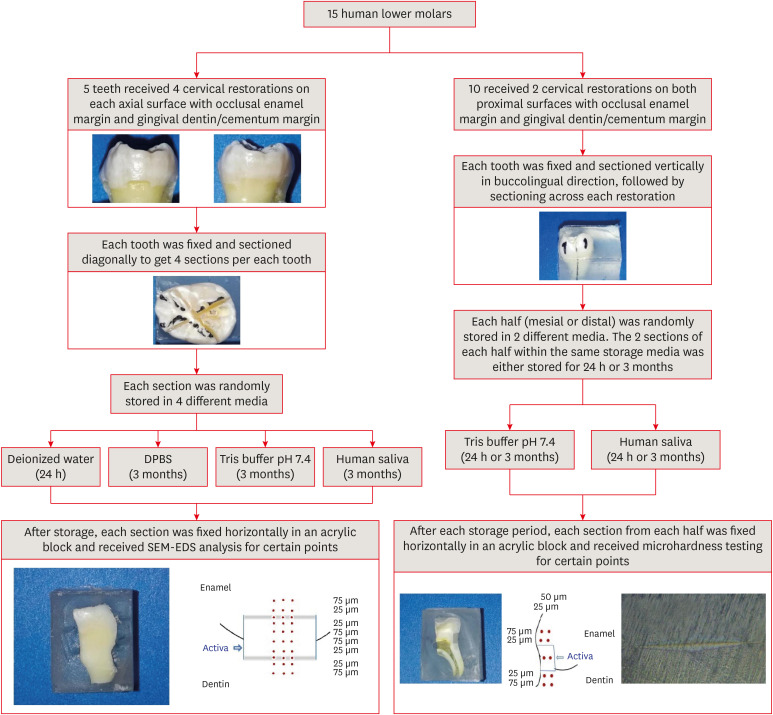
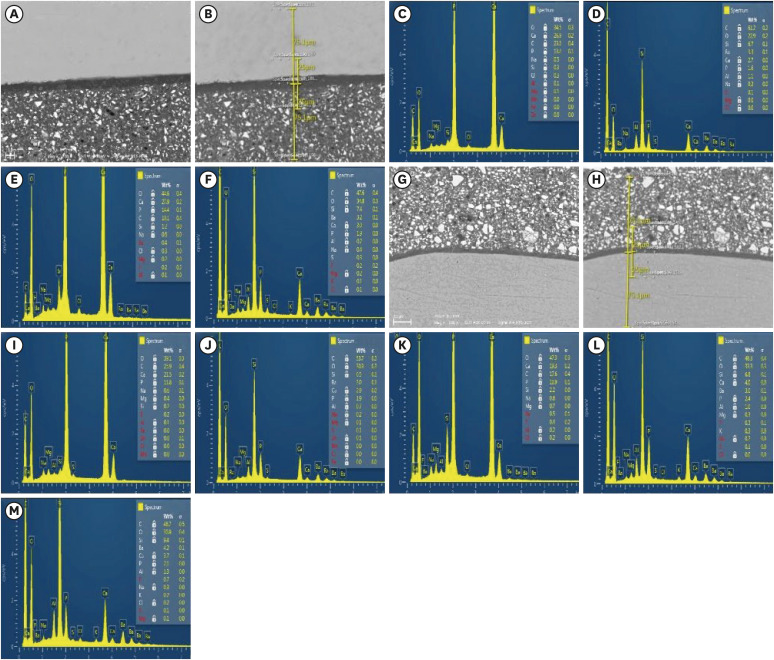
Fifteen teeth received cervical restorations with occlusal enamel and gingival dentin margins using the tested material bonded with a universal adhesive, 5 of them on the 4 axial surfaces and the other 10 on only the 2 proximal surfaces. The first 5 teeth were sectioned into 4 restorations each, then stored in 4 different media; deionized water, Dulbecco's phosphate buffered saline (DPBS), Tris buffer, and saliva. The storage period for deionized water was 24 hours while it was 3 months for the other media. Each part was analyzed by scanning electron microscopy-energy dispersive spectroscopy (SEM-EDS) analysis for different substrates/distances and the wt% of calcium, phosphorus, silica, and fluoride were calculated. The other 10 teeth were sectioned across the restoration, stored in either Tris buffer or saliva for 24 hours or 3 months, and were evaluated for microhardness of different substrates/ areas. Data were analyzed using analysis of variance and Tukey’s post hoc test.
Results
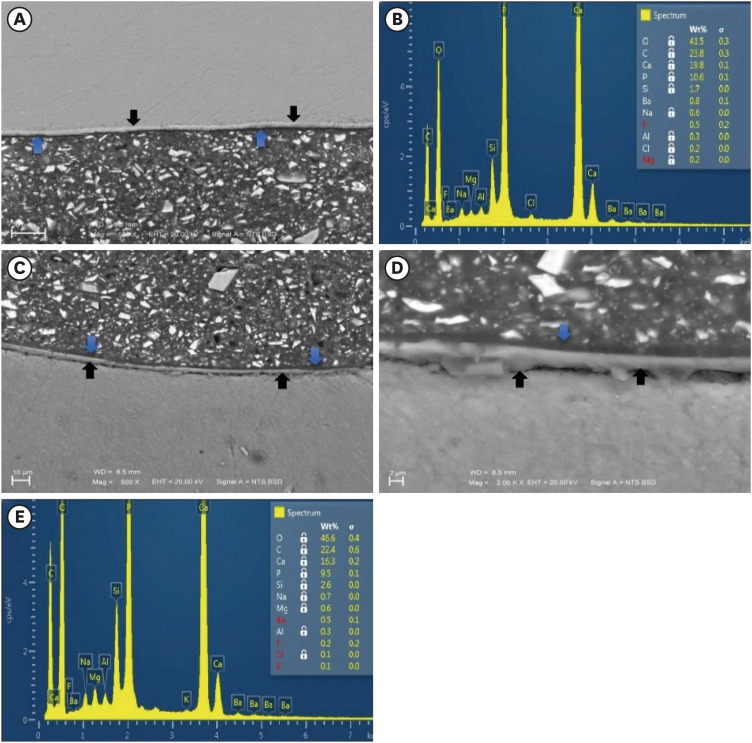
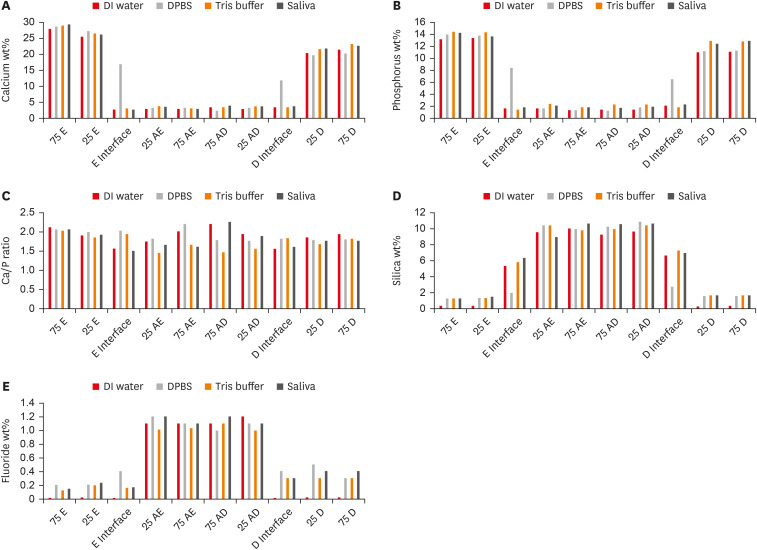
Enamel and dentin interfaces in the DPBS group exhibited a significant increase in calcium and phosphorus wt%. Both silica and fluoride significantly increased in tooth structure up to a distance of 75 μm in the 3-month-media groups than the immediate group. Storage media did not affect the microhardness values.
Conclusions
SEM-EDS analysis suggests an ion movement between Activa and tooth structure through a universal adhesive while stored in DPBS.
Keyword
Figure
Reference
-
1. Tiskaya M, Al-Eesa NA, Wong FS, Hill RG. Characterization of the bioactivity of two commercial composites. Dent Mater. 2019; 35:1757–1768. PMID: 31699444.2. Jefferies SR, Fuller AE, Boston DW. Preliminary evidence that bioactive cements occlude artificial marginal gaps. J Esthet Restor Dent. 2015; 27:155–166. PMID: 25640821.3. Lai G, Li M. Secondary caries. Li MY, editor. Contemporary approach to dental caries. Rijeka, Croatia: InTech;2012.4. Cenci MS, Pereira-Cenci T, Cury JA, Ten Cate JM. Relationship between gap size and dentine secondary caries formation assessed in a microcosm biofilm model. Caries Res. 2009; 43:97–102. PMID: 19321986.5. Vallittu PK, Boccaccini AR, Hupa L, Watts DC. Bioactive dental materials-Do they exist and what does bioactivity mean? Dent Mater. 2018; 34:693–694. PMID: 29571660.6. Gandolfi MG, Taddei P, Tinti A, Dorigo ED, Prati C. Alpha-TCP improves the apatite-formation ability of calcium-silicate hydraulic cement soaked in phosphate solutions. Mater Sci Eng C. 2011; 31:1412–1422.7. Ruengrungsom C, Burrow MF, Parashos P, Palamara JE. Evaluation of F, Ca, and P release and microhardness of eleven ion-leaching restorative materials and the recharge efficacy using a new Ca/P containing fluoride varnish. J Dent. 2020; 102:103474. PMID: 32941973.8. Gandolfi MG, Ciapetti G, Taddei P, Perut F, Tinti A, Cardoso MV, et al. Apatite formation on bioactive calcium-silicate cements for dentistry affects surface topography and human marrow stromal cells proliferation. Dent Mater. 2010; 26:974–992. PMID: 20655582.9. Fuss M, Wicht MJ, Attin T, Derman SH, Noack MJ. Protective buffering capacity of restorative dental materials in vitro . J Adhes Dent. 2017; 19:177–183. PMID: 28443832.10. Kirsten GA, Rached RN, Mazur RF, Vieira S, Souza EM. Effect of open-sandwich vs. adhesive restorative techniques on enamel and dentine demineralization: an in situ study. J Dent. 2013; 41:872–880. PMID: 23851134.11. Kirsten GA, Takahashi MK, Rached RN, Giannini M, Souza EM. Microhardness of dentin underneath fluoride-releasing adhesive systems subjected to cariogenic challenge and fluoride therapy. J Dent. 2010; 38:460–468. PMID: 20193726.12. Al-Harbi F, Kaisarly D, Michna A, ArRejaie A, Bader D, El Gezawi M. Cervical interfacial bonding effectiveness of class II bulk versus incremental fill resin composite restorations. Oper Dent. 2015; 40:622–635. PMID: 26151459.13. Irie M, Suzuki K, Watts DC. Delayed polishing technique on glass–ionomer restorations. Jpn Dent Sci Rev. 2009; 45:14–22.14. Magura ME, Kafrawy AH, Brown CE Jr, Newton CW. Human saliva coronal microleakage in obturated root canals: an in vitro study. J Endod. 1991; 17:324–331. PMID: 1779218.15. Moheet IA, Luddin N, Ab Rahman I, Masudi SM, Kannan TP, Nik Abd Ghani NR. Analysis of ionic-exchange of selected elements between novel nano-hydroxyapatite-silica added glass ionomer cement and natural teeth. Polymers (Basel). 2021; 13:3504. PMID: 34685263.16. Thakur AK, Srivastava N, Chakrabarty T, Rebary B, Patidar R, Sanghavi RJ, et al. An improved protocol for electrodialytic desalination yielding mineral-balanced potable water. Desalination. 2014; 335:96–101.17. Parirokh M, Askarifard S, Mansouri S, Haghdoost AA, Raoof M, Torabinejad M. Effect of phosphate buffer saline on coronal leakage of mineral trioxide aggregate. J Oral Sci. 2009; 51:187–191. PMID: 19550085.18. Han L, Okiji T, Okawa S. Morphological and chemical analysis of different precipitates on mineral trioxide aggregate immersed in different fluids. Dent Mater J. 2010; 29:512–517. PMID: 20823620.19. Zain S, Davis GR, Hill R, Anderson P, Baysan A. Mineral exchange within restorative materials following incomplete carious lesion removal using 3D non-destructive XMT subtraction methodology. J Dent. 2020; 99:103389. PMID: 32492503.20. Chun K, Choi H, Lee J. Comparison of mechanical property and role between enamel and dentin in the human teeth. J Dent Biomech. 2014; 5:1758736014520809. PMID: 24550998.21. Nicholson JW. Glass ionomer dental cements: update. Mater Technol. 2010; 25:8–13.22. Ngo H, Mount GJ, Peters MC. A study of glass-ionomer cement and its interface with enamel and dentin using a low-temperature, high-resolution scanning electron microscopic technique. Quintessence Int. 1997; 28:63–69. PMID: 10332357.23. Sauro S, Makeeva I, Faus-Matoses V, Foschi F, Giovarruscio M, Maciel Pires P, et al. Effects of ions-releasing restorative materials on the dentine bonding longevity of modern universal adhesives after load-cycle and prolonged artificial saliva aging. Materials (Basel). 2019; 12:722. PMID: 30832247.24. Tay FR, Pashley DH, Suh B, Carvalho R, Miller M. Single-step, self-etch adhesives behave as permeable membranes after polymerization. Part I. Bond strength and morphologic evidence. Am J Dent. 2004; 17:271–278. PMID: 15478490.25. Itthagarun A, Tay FR, Pashley DH, Wefel JS, García-Godoy F, Wei SH. Single-step, self-etch adhesives behave as permeable membranes after polymerization. Part III. Evidence from fluid conductance and artificial caries inhibition. Am J Dent. 2004; 17:394–400. PMID: 15724748.26. Gonçalves LL, Da Silva TM, Prakki A, Barcellos DC, Caneppele TM, De Oliveira HP, et al. Universal adhesive: the effect of different simulated pulpal pressure fluids and bonding modes to dentin. Odontology. 2022; 110:62–69. PMID: 34213683.27. Chen C, Niu LN, Xie H, Zhang ZY, Zhou LQ, Jiao K, et al. Bonding of universal adhesives to dentine--old wine in new bottles? J Dent. 2015; 43:525–536. PMID: 25797702.28. Cruz J, Silva A, Eira R, Sousa B, Lopes M, Cavalheiro A. Dentin permeability and nanoleakage of universal adhesives in etch-and-rinse vs self-etch modes. Oper Dent. 2021; 46:293–305. PMID: 34424991.29. Pires PM, Neves AA, Makeeva IM, Schwendicke F, Faus-Matoses V, Yoshihara K, et al. Contemporary restorative ion-releasing materials: current status, interfacial properties and operative approaches. Br Dent J. 2020; 229:450–458. PMID: 33037365.30. Yamamoto H, Iwami Y, Unezaki T, Tomii Y, Ebisu S. Fluoride uptake in human teeth from fluoride-releasing restorative material in vivo and in vitro: two-dimensional mapping by EPMA-WDX. Caries Res. 2001; 35:111–115. PMID: 11275670.31. Wiegand A, Buchalla W, Attin T. Review on fluoride-releasing restorative materials--fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent Mater. 2007; 23:343–362. PMID: 16616773.32. Gašperšič D. Enamel microhardness and histological features of composite enamel pearls of different size. J Oral Pathol Med. 1995; 24:153–158. PMID: 7783004.33. Jang JH, Lee MG, Ferracane JL, Davis H, Bae HE, Choi D, et al. Effect of bioactive glass-containing resin composite on dentin remineralization. J Dent. 2018; 75:58–64. PMID: 29807059.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effect of restorative materials on the stress distribution of class V composite resin restorations: a 3D finite element investigation
- Influence of Storage Temperature on Levels of Bioactive Compounds in Shiitake Mushrooms (Lentinula edodes)
- Effect of three nanobiomaterials on microhardness of bleached enamel
- Effect of organic acids in dental biofilm on microhardness of a silorane-based composite
- The effect of marginal microleakge according to thickness of flowable resin