J Rhinol.
2024 Mar;31(1):17-21. 10.18787/jr.2023.00066.
Insufficiency of Laboratory Data in Reflecting Allergic Rhinitis Severity Based on the Allergic Rhinitis and Its Impact on Asthma Guideline in Korean Patients
- Affiliations
-
- 1Department of Otorhinolaryngology, Yonsei University College of Medicine, Seoul, Republic of Korea
- 2Severance Biomedical Science Institute, Yonsei University College of Medicine, Seoul, Republic of Korea
- 3The Airway Mucus Institute, Yonsei University College of Medicine, Severance Hospital, Seoul, Republic of Korea
- KMID: 2554086
- DOI: http://doi.org/10.18787/jr.2023.00066
Abstract
- Background and Objectives
This retrospective study, conducted at a single tertiary medical center, aimed to investigate the correlation between the severity of allergic rhinitis (AR) based on subjective symptoms and the severity assessed through laboratory data.
Methods
In total, 584 patients who were diagnosed with AR by a multiple-allergen simultaneous test were included. Patients were classified into four groups according to the Allergic Rhinitis and its Impact on Asthma (ARIA) classification guideline. The visual analog scale (VAS) score for overall discomfort and laboratory parameters, including the serum total immunoglobulin E (IgE) level and peripheral blood eosinophil count, were evaluated in all patients. An analysis was conducted to examine the differences in VAS scores and laboratory findings among the four groups. Additionally, the correlations between the laboratory findings and VAS score were analyzed.
Results
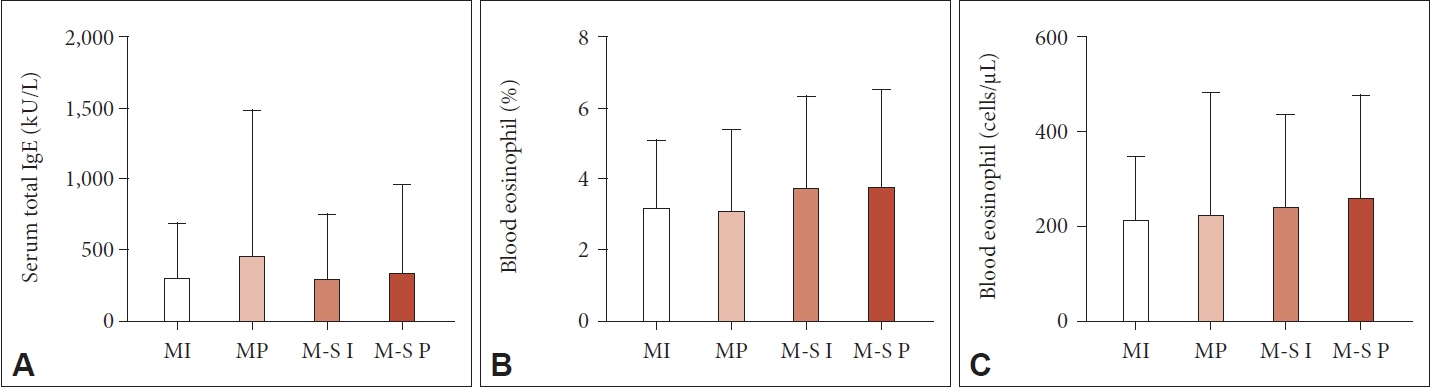
The serum total IgE level and the percentage and count of peripheral blood eosinophils showed no significant differences among the groups. However, the VAS score for overall discomfort exhibited notable between-group differences. The average VAS score was 6.14 (95% confidence interval 5.94–6.34) in the overall group. The mean scores of each group showed a noticeable increasing trend from the mild intermittent group to the mild persistent, moderate to severe intermittent, and moderate to severe persistent groups (p<0.001), although there was no clear correlation between the increase in VAS scores and laboratory parameters.
Conclusion
Neither the symptom-based ARIA guideline nor the VAS score correlated with the AR laboratory test measurements. The current laboratory data alone may not be sufficient to reflect the severity of AR based on subjective symptoms.
Figure
Reference
-
References
1. Bousquet PJ, Leynaert B, Neukirch F, Sunyer J, Janson CM, Anto J, et al. Geographical distribution of atopic rhinitis in the European Community Respiratory Health Survey I. Allergy. 2008; 63(10):1301–9.
Article2. Bousquet J, Anto JM, Bachert C, Baiardini I, Bosnic-Anticevich S, Walter Canonica G, et al. Allergic rhinitis. Nat Rev Dis Primers. 2020; 6(1):95.
Article3. Vandenplas O, Vinnikov D, Blanc PD, Agache I, Bachert C, Bewick M, et al. Impact of rhinitis on work productivity: a systematic review. J Allergy Clin Immunol Pract. 2018; 6(4):1274–86.e9.
Article4. Devillier P, Bousquet J, Salvator H, Naline E, Grassin-Delyle S, de Beaumont O. In allergic rhinitis, work, classroom and activity impairments are weakly related to other outcome measures. Clin Exp Allergy. 2016; 46(11):1456–64.
Article5. Vlaminck S, Acke F, Scadding GK, Lambrecht BN, Gevaert P. Pathophysiological and clinical aspects of chronic rhinosinusitis: current concepts. Front Allergy. 2021; 2:741788.
Article6. Eschenbacher W, Straesser M, Knoeddler A, Li RC, Borish L. Biologics for the treatment of allergic rhinitis, chronic rhinosinusitis, and nasal polyposis. Immunol Allergy Clin North Am. 2020; 40(4):539–47.
Article7. Lee CH, Jang JH, Lee HJ, Kim IT, Chu MJ, Kim CD, et al. Clinical characteristics of allergic rhinitis according to Allergic Rhinitis and its Impact on Asthma guidelines. Clin Exp Otorhinolaryngol. 2008; 1(4):196–200.
Article8. Bousquet J, Schünemann HJ, Hellings PW, Arnavielhe S, Bachert C, Bedbrook A, et al. MACVIA clinical decision algorithm in adolescents and adults with allergic rhinitis. J Allergy Clin Immunol. 2016; 138(2):367–74.e2.9. Bousquet J, Schünemann HJ, Togias A, Bachert C, Erhola M, Hellings PW, et al. Next-generation allergic rhinitis and its impact on asthma (ARIA) guidelines for allergic rhinitis based on grading of recommendations assessment, development and evaluation (GRADE) and real-world evidence. J Allergy Clin Immunol. 2020; 145(1):70–80.e3. .10. Bousquet J, Van Cauwenberge P, Khaltaev N; Aria Workshop Group; World Health Organization. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001; 108(5 Suppl):S147–334.
Article11. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic rhinitis and its impact on asthma (ARIA) 2008. Allergy. 2008; 63(Suppl 86):8–160.12. Bousquet PJ, Combescure C, Neukirch F, Klossek JM, Méchin H, Daures JP, et al. Visual analog scales can assess the severity of rhinitis graded according to ARIA guidelines. Allergy. 2007; 62(4):367–72.
Article13. Bousquet PJ, Bachert C, Canonica GW, Casale TB, Mullol J, Klossek JM, et al. Uncontrolled allergic rhinitis during treatment and its impact on quality of life: a cluster randomized trial. J Allergy Clin Immunol. 2010; 126(3):666–8.e5.14. Sensi LG, Piacentini GL, Nobile E, Ghebregzabher M, Brunori R, Zanolla L, et al. Changes in nasal specific IgE to mites after periods of allergen exposure-avoidance: a comparison with serum levels. Clin Exp Allergy. 1994; 24(4):377–82.
Article15. KleinJan A, Godthelp T, van Toornenenbergen AW, Fokkens WJ. Allergen binding to specific IgE in the nasal mucosa of allergic patients. J Allergy Clin Immunol. 1997; 99(4):515–21.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Allergic Rhinitis and Its Impact on Asthma Update (ARIA 2008) Korean Perspective
- Clinical Practice Guideline for Physicians on Allergic Rhinitis
- Diagnosis of Allergic Rhinitis
- Allergic rhinitis, sinusitis and asthma: evidence for respiratory system integration
- Skin Prick Testing of Patients with Allergic Rhinitis and/or Asthma: a Study in Catholic Medical Center, Korea