J Liver Cancer.
2024 Mar;24(1):71-80. 10.17998/jlc.2023.09.11.
Treatment options for solitary hepatocellular carcinoma ≤5 cm: surgery vs. ablation: a multicenter retrospective study
- Affiliations
-
- 1Department of Gastroenterology, Okayama City Civic Hospital, Okayama, Japan
- 2Gastroenterology Center, Ehime Prefectural Central Hospital, Matsuyama, Japan
- 3Department of Gastroenterology and Hepatology, Ogaki Municipal Hospital, Ogaki, Japan
- 4Department of Internal Medicine, Japanese Red Cross Society Himeji Hospital, Himeji, Japan
- 5Center of Gastroenterology, Teine Keijinkai Hospital, Sapporo, Japan
- 6Department of Gastroenterology, Saiseikai Niigata Hospital, Niigata, Japan
- 7Department of Gastroenterology, Gunma Saiseikai Maebashi Hospital, Maebashi, Japan
- 8Department of Gastroenterology, Asahi General Hospital, Asahi, Japan
- 9Department of Hepatology, Kagawa Prefectural Central Hospital, Takamatsu, Japan
- 10Department of Gastroenterology, National Hospital Organization Takasaki General Medical Center, Takasaki, Japan
- 11Department of Clinical Research, National Hospital Organization Takasaki General Medical Center, Takasaki, Japan
- 12Department of Gastroenterology and Hepatology, Kindai University Faculty of Medicine, Higashiosaka, Japan
- 13Department of Nursing, Gifu Kyōritsu University, Ogaki, Japan
- KMID: 2553819
- DOI: http://doi.org/10.17998/jlc.2023.09.11
Abstract
- Background
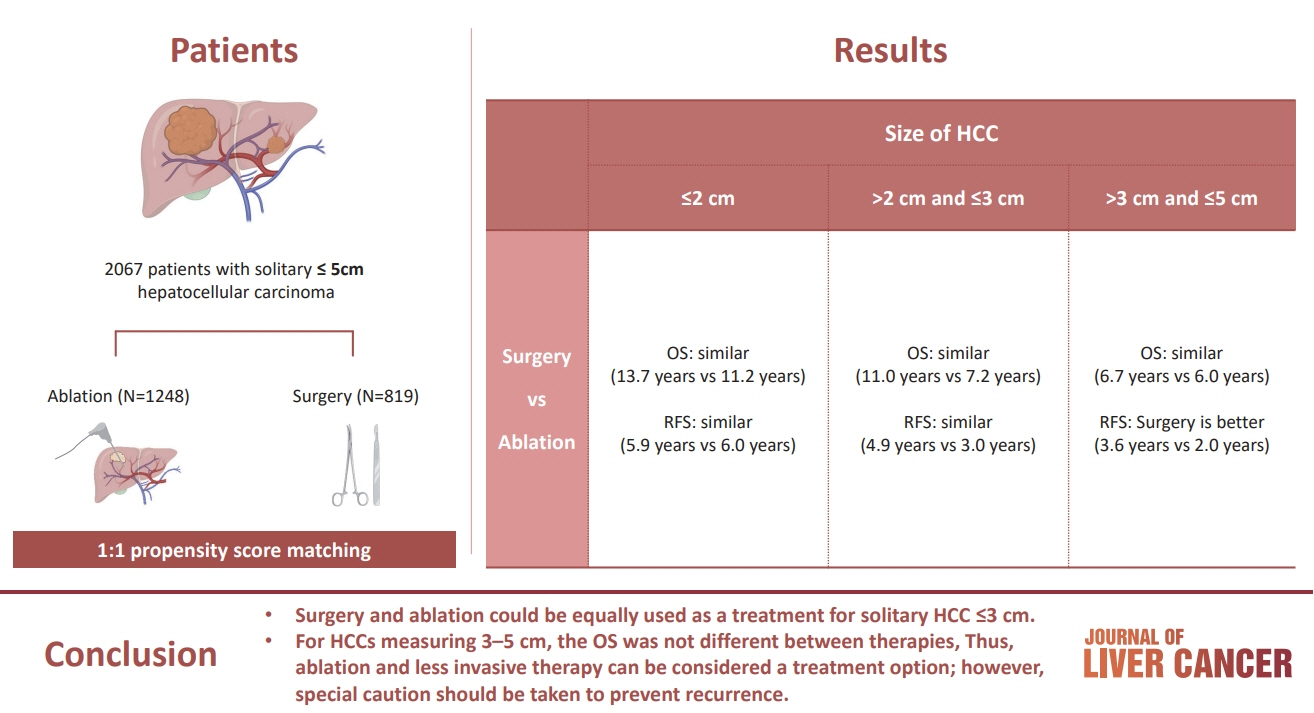
/Aim: The aim of this study was to compare the therapeutic efficacy of ablation and surgery in solitary hepatocellular carcinoma (HCC) measuring ≤5 cm with a large HCC cohort database.
Methods
The study included consecutive 2,067 patients with solitary HCC who were treated with either ablation (n=1,248) or surgery (n=819). Th e patients were divided into three groups based on the tumor size and compared the outcomes of the two therapies using propensity score matching.
Results
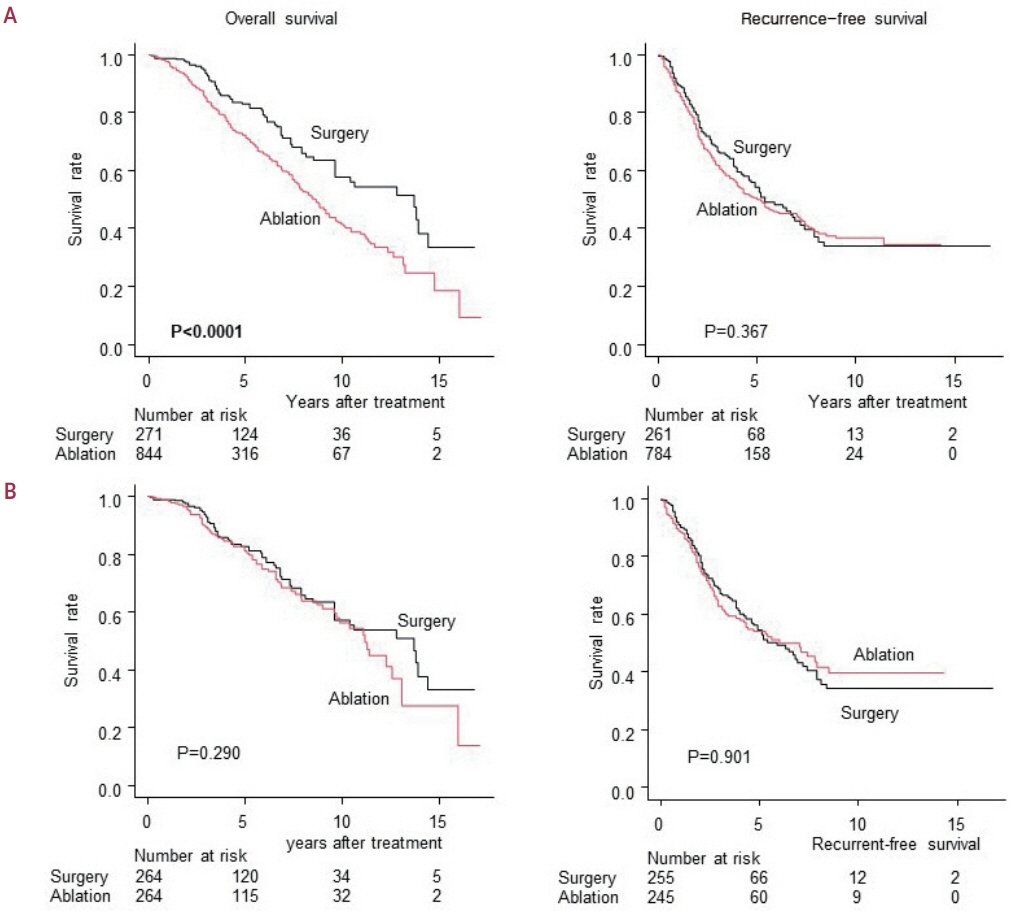
No significant difference in recurrence-free survival (RFS) or overall survival (OS) was found between surgery and ablation groups for tumors measuring ≤2 cm or >2 cm but ≤3 cm. For tumors measuring >3 cm but ≤5 cm, RFS was significantly better with surgery than with ablation (3.6 and 2.0 years, respectively, P=0.0297). However, no significant difference in OS was found between surgery and ablation in this group (6.7 and 6.0 years, respectively, P=0.668).
Conclusion
The study suggests that surgery and ablation can be equally used as a treatment for solitary HCC no more than 3 cm in diameter. For HCCs measuring 3-5 cm, the OS was not different between therapies; thus, ablation and less invasive therapy can be considered a treatment option; however, special caution should be taken to prevent recurrence.
Figure
Cited by 3 articles
-
Radiofrequency for hepatocellular carcinoma larger than 3 cm: potential for applications in daily practice
Ji Hoon Kim, Pil Soo Sung
J Liver Cancer. 2024;24(1):1-2. doi: 10.17998/jlc.2024.02.13.Letter regarding “Treatment options for solitary hepatocellular carcinoma ≤5 cm: surgery vs. ablation: a multicenter retrospective study”
Jongman Kim
J Liver Cancer. 2024;24(1):3-4. doi: 10.17998/jlc.2023.12.04.Reply to the Letter regarding “Treatment options for solitary hepatocellular carcinoma ≤5 cm: surgery vs. ablation: a multicenter retrospective study”
Kazuhiro Nouso, Kazuya Kariyama
J Liver Cancer. 2024;24(1):5-6. doi: 10.17998/jlc.2024.01.02.
Reference
-
References
1. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008; 359:378–390.
Article2. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018; 391:1163–1173.
Article3. Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebocontrolled, phase 3 trial. Lancet. 2017; 389:56–66.
Article4. Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019; 20:282–296.
Article5. Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018; 379:54–63.
Article6. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020; 382:1894–1905.
Article7. Kelley RK, Sangro B, Harris W, Ikeda M, Okusaka T, Kang YK, et al. Safety, efficacy, and pharmacodynamics of tremelimumab plus durvalumab for patients with unresectable hepatocellular carcinoma: randomized expansion of a phase I/II study. J Clin Oncol. 2021; 39:2991–3001.
Article8. Ng KKC, Chok KSH, Chan ACY, Cheung TT, Wong TCL, Fung JYY, et al. Randomized clinical trial of hepatic resection versus radiofrequency ablation for early-stage hepatocellular carcinoma. Br J Surg. 2017; 104:1775–1784.
Article9. Hsiao CY, Hu RH, Ho CM, Wu YM, Lee PH, Ho MC. Surgical resection versus radiofrequency ablation for Barcelona Clinic Liver Cancer very early stage hepatocellular carcinoma: long-term results of a singlecenter study. Am J Surg. 2020; 220:958–964.
Article10. Imai K, Beppu T, Chikamoto A, Doi K, Okabe H, Hayashi H, et al. Comparison between hepatic resection and radiofrequency ablation as firstline treatment for solitary small-sized hepatocellular carcinoma of 3 cm or less. Hepatol Res. 2013; 43:853–864.
Article11. Zhang T, Hu H, Jia Y, Gao Y, Hao F, Wu J, et al. Efficacy and safety of radiofrequency ablation and surgery for hepatocellular carcinoma in patients with cirrhosis: a meta-analysis. Medicine (Baltimore). 2022; 101:e32470.
Article12. Ivanics T, Rajendran L, Abreu PA, Claasen MPAW, Shwaartz C, Patel MS, et al. Long-term outcomes of ablation, liver resection, and liver transplant as first-line treatment for solitary HCC of 3 cm or less using an intention-to-treat analysis: a retrospective cohort study. Ann Med Surg (Lond). 2022; 77:103645.
Article13. Zheng L, Zhang CH, Lin JY, Song CL, Qi XL, Luo M. Comparative effectiveness of radiofrequency ablation vs. surgical resection for patients with solitary hepatocellular carcinoma smaller than 5 cm. Front Oncol. 2020; 10:399.
Article14. Ko SE, Lee MW, Ahn S, Rhim H, Kang TW, Song KD, et al. Laparoscopic hepatic resection versus laparoscopic radiofrequency ablation for subcapsular hepatocellular carcinomas smaller than 3 cm: analysis of treatment outcomes using propensity score matching. Korean J Radiol. 2022; 23:615–624.
Article15. Takayama T, Hasegawa K, Izumi N, Kudo M, Shimada M, Yamanaka N, et al. Surgery versus radiofrequency ablation for small hepatocellular carcinoma: a randomized controlled trial (SURF trial). Liver Cancer. 2021; 11:209–218.
Article16. Kudo M, Kawamura Y, Hasegawa K, Tateishi R, Kariyama K, Shiina S, et al. Management of hepatocellular carcinoma in Japan: JSH consensus statements and recommendations 2021 update. Liver Cancer. 2021; 10:181–223.
Article17. Thamtorawat S, Hicks RM, Yu J, Siripongsakun S, Lin WC, Raman SS, et al. Preliminary outcome of microwave ablation of hepatocellular carcinoma: breaking the 3-cm barrier? J Vasc Interv Radiol. 2016; 27:623–630.
Article18. Lee HW, Lee JM, Yoon JH, Kim YJ, Park JW, Park SJ, et al. A prospective randomized study comparing radiofrequency ablation and hepatic resection for hepatocellular carcinoma. Ann Surg Treat Res. 2018; 94:74–82.
Article19. Kudo M, Kitano M, Sakurai T, Nishida N. General rules for the clinical and pathological study of primary liver cancer, nationwide follow-up survey and clinical practice guidelines: the outstanding achievements of the Liver Cancer Study Group of Japan. Dig Dis. 2015; 33:765–770.
Article20. Liver Cancer Study Group of Japan. The general rules for the clinical and pathological study of primary liver cancer, 6th ed. Tokyo: Kanehara, 2015:26-30.21. Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011; 10:150–161.
Article22. Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013; 48:452–458.
Article23. R Core Team. The R project for statistical computing, 2023 [Internet]. Vienna (AT): R Foundation for Statistical Computing; [cited 2023 Aug 5]. Available from: https://www.R-project.org/.24. Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006; 243:321–328.
Article25. Wang Z, Liu M, Zhang DZ, Wu SS, Hong ZX, He GB, et al. Microwave ablation versus laparoscopic resection as first-line therapy for solitary 3-5- cm HCC. Hepatology. 2022; 76:66–77.
Article26. Korean Liver Cancer Association (KLCA); National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Clin Mol Hepatol. 2022; 28:583–705.27. Shao YY, Wang SY, Lin SM; Diagnosis Group; Systemic Therapy Group. Management consensus guideline for hepatocellular carcinoma: 2020 update on surveillance, diagnosis, and systemic treatment by the Taiwan Liver Cancer Association and the Gastroenterological Society of Taiwan. J Formos Med Assoc. 2021; 120:1051–1060.
Article28. Minami Y, Kudo M. Image guidance in ablation for hepatocellular carcinoma: contrast-enhanced ultrasound and fusion imaging. Front Oncol. 2021; 11:593636.
Article29. Minami Y, Minami T, Hagiwara S, Ida H, Ueshima K, Nishida N, et al. Ultrasound-ultrasound image overlay fusion improves real-time control of radiofrequency ablation margin in the treatment of hepatocellular carcinoma. Eur Radiol. 2018; 28:1986–1993.
Article30. Nishimura M, Nouso K, Kariyama K, Wakuta A, Kishida M, Wada N, et al. Safety and efficacy of radiofrequency ablation with artificial ascites for hepatocellular carcinoma. Acta Med Okayama. 2012; 66:279–284.31. Uehara T, Hirooka M, Ishida K, Hiraoka A, Kumagi T, Kisaka Y, et al. Percutaneous ultrasound-guided radiofrequency ablation of hepatocellular carcinoma with artificially induced pleural effusion and ascites. J Gastroenterol. 2007; 42:306–311.
Article32. Kariyama K, Nouso K, Wakuta A, Kishida M, Nishimura M, Wada N, et al. Percutaneous radiofrequency ablation for treatment of hepatocellular carcinoma in the caudate lobe. AJR Am J Roentgenol. 2011; 197:W571–W575.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Letter regarding “Treatment options for solitary hepatocellular carcinoma ≤5 cm: surgery vs. ablation: a multicenter retrospective study”
- Reply to the Letter regarding “Treatment options for solitary hepatocellular carcinoma ≤5 cm: surgery vs. ablation: a multicenter retrospective study”
- The Role of Combination of Transarterial Chemoebolization and Radiofrequency Ablation for Hepatocellular Carcinoma Treatment
- Completely Ablated Hepatocellular Carcinoma by Percutaneous Radiofrequency Thermal Ablation
- Radiofrequency Thermal Ablation of Hepatocellular Carcinomas