J Dent Rehabil Appl Sci.
2024 Feb;40(1):1-12. 10.14368/jdras.2024.40.1.1.
Effect of calcium silicate-based sealer to bone tissue of mandible of rats
- Affiliations
-
- 1Department of Conservative Dentistry, College of Dentistry, Gangneung-Wonju National University, Gangneung, Republic of Korea
- 2Department of Anatomy, College of Dentistry, Gangneung-Wonju National University, Gangneung, Republic of Korea
- KMID: 2553626
- DOI: http://doi.org/10.14368/jdras.2024.40.1.1
Abstract
- Purpose
To histologically evaluate the effects of three calcium silicate-based sealers on rat mandible tissue.
Materials and Methods
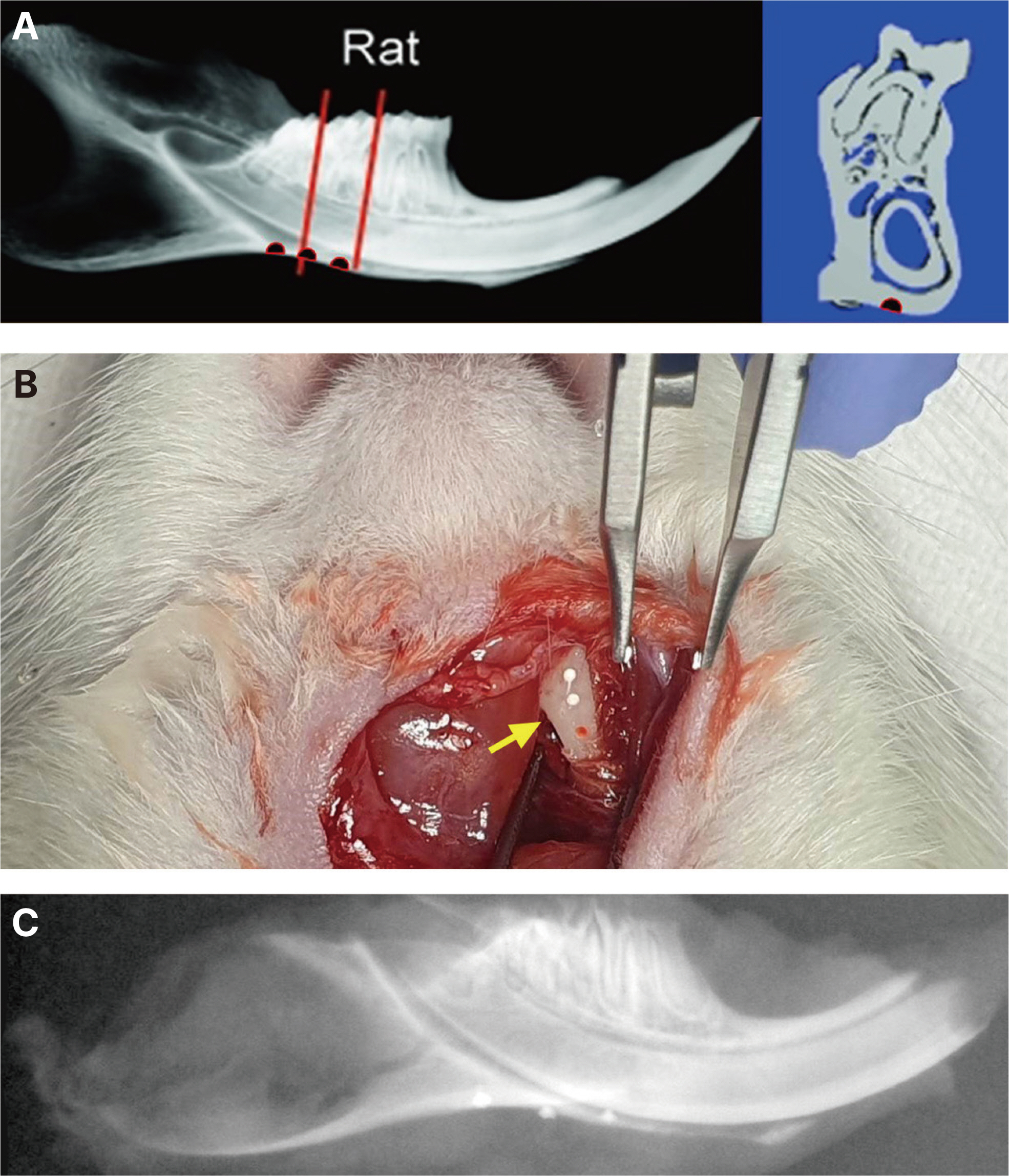
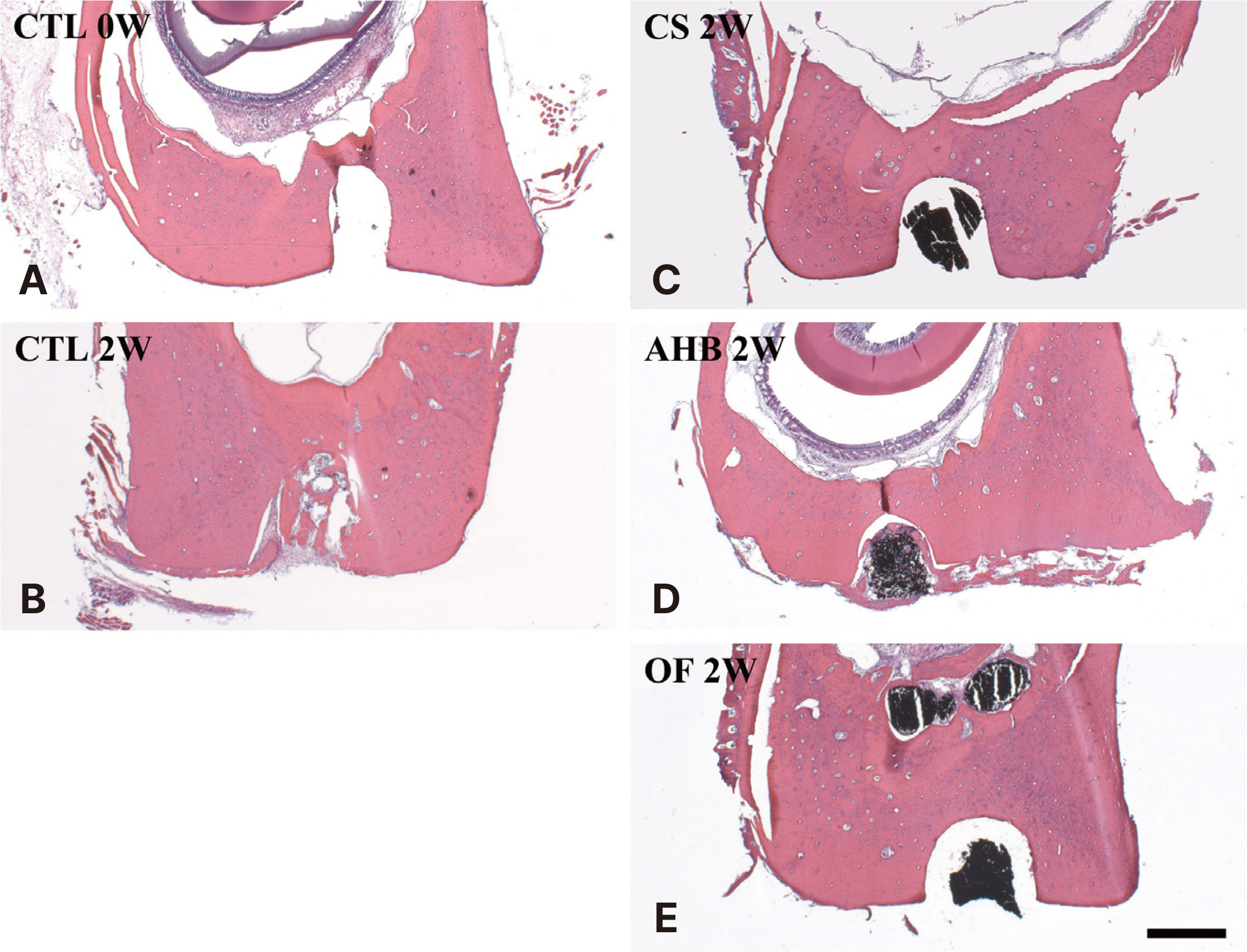
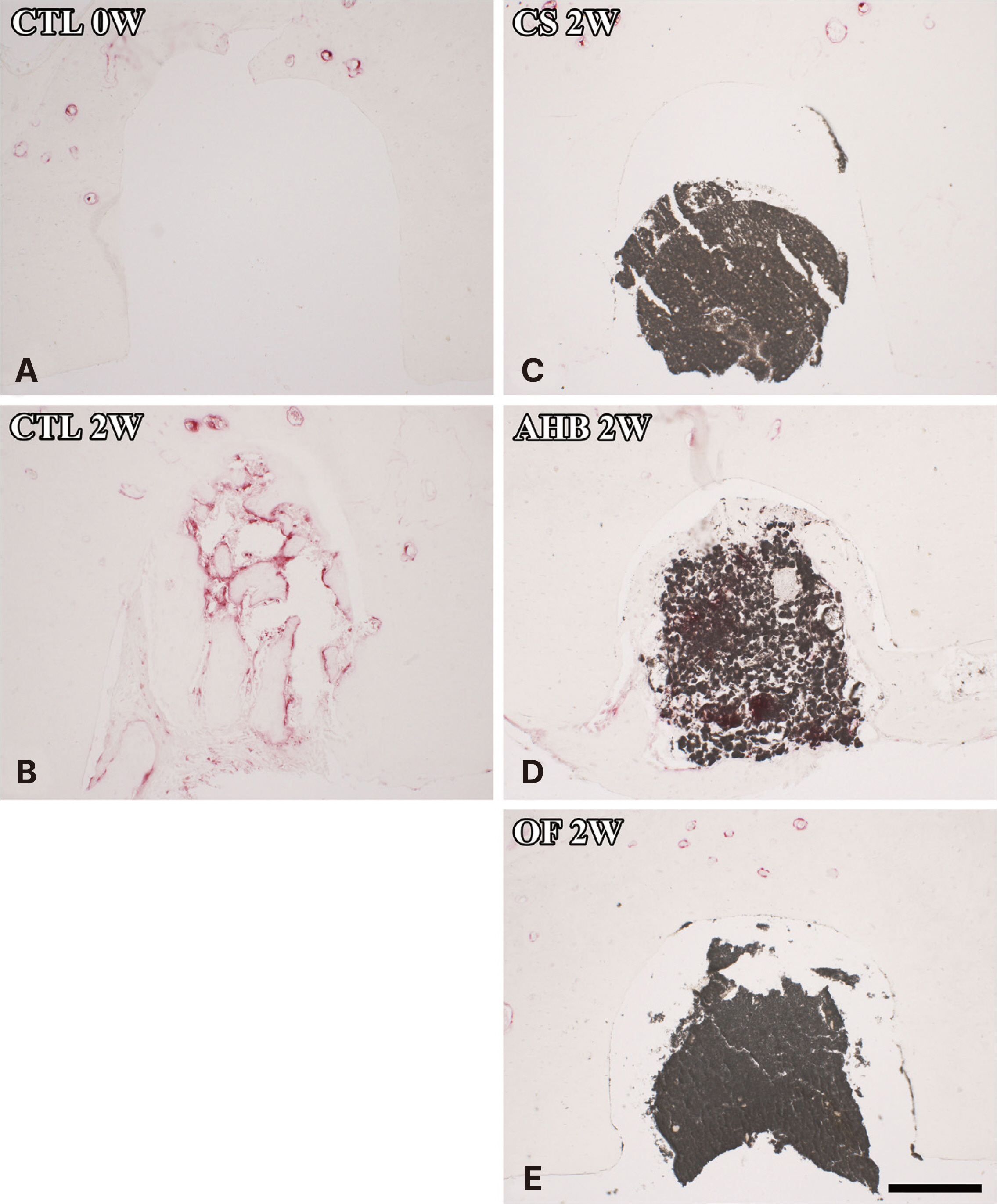
Rats were randomly divided as follows: A group that sacrificed immediately after cavity preparation, a group that sacri-ficed two weeks after cavity preparation, a group that sacrificed two weeks after CeraSeal (CS), AH Plus Bioceramic (AHB), or OneFil (OF) sealer injection, respectively. After tissue processing for all groups, the bone tissue area (%) and the number of osteoclasts in and around the cavity were measured under a microscope. The results of each group were compared and statistical analysis was performed using one-way ANOVA and Tukey’s test.
Results
The formation of bone tissue and the presence of osteoclasts in the cav-ity were observed in the group that sacrificed two weeks after cavity preparation and the group sacrificed two weeks after AHB seal-er injection, and these groups showed significantly higher average bone tissue area (%) than the other groups. In the other groups, no inflammation or foreign body reaction occurred in the cavity, and no osteoclasts were observed.
Conclusion
All calcium silicatebased sealers used in this study showed a favorable bone tissue response when injected into the rat mandible. In particular, higher bone formation in the cavity was observed in AHB.
Figure
Reference
-
References
1. Friedman S, Torneck CD, Komorowski R, Ouzounian Z, Syrtash P, Kaufman A. 1997; In vivo model for assessing the functional efficacy of endodontic filling materials and techniques. J Endod. 23:557–61. DOI: 10.1016/S0099-2399(06)81120-9. PMID: 9587280.
Article2. Ng YL, Mann V, Rahbaran S, Lewsey J, Gulabivala K. 2008; Outcome of primary root canal treatment: systematic review of the literature-Part 2. Influence of clinical factors. Int Endod J. 41:6–31. DOI: 10.1111/j.1365-2591.2007.01323.x. PMID: 17931388.
Article3. Onay EO, Ungor M, Orucoglu H. 2006; An in vitro evaluation of the apical sealing ability of a new resin-based root canal obturation system. J Endod. 32:976–8. DOI: 10.1016/j.joen.2006.05.013. PMID: 16982277.
Article4. Santos JM, Pereira S, Sequeira DB, Messias AL, Martins JB, Cunha H, Palma PJ, Santos AC. 2019; Biocompatibility of a bioceramic silicone-based sealer in subcutaneous tissue. J Oral Sci. 61:171–7. DOI: 10.2334/josnusd.18-0145. PMID: 30918214.
Article5. Gluskin AH. 2005; Mishaps and serious complications in endodontic obturation. Endod Topics. 12:52–70. DOI: 10.1111/j.1601-1546.2005.00194.x.
Article6. Holland R, Sant'anna Júnior A, de Souza V, Dezan Junior E, Otoboni Filho JA, Bernabé BF, Nery MJ, Murata SS. 2005; Influence of apical patency and filling material on healing process of dogs' teeth with vital pulp after root canal therapy. Braz Dent J. 16:9–16. DOI: 10.1590/S0103-64402005000100002. PMID: 16113927.
Article7. Goldberg F, Cantarini C, Alfie D, Macchi RL, Arias A. 2020; Relationship between unintentional canal overfilling and the long-term outcome of primary root canal treatments and nonsurgical retreatments: a retrospective radiographic assessment. Int Endod J. 53:19–26. DOI: 10.1111/iej.13209. PMID: 31454090.
Article8. Chybowski EA, Glickman GN, Patel Y, Fleury A, Solomon E, He J. 2018; Clinical outcome of non-surgical root canal treatment using a single-cone technique with endosequence bioceramic sealer: a retrospective analysis. J Endod. 44:941–5. DOI: 10.1016/j.joen.2018.02.019. PMID: 29606401.
Article9. Dos Santos Costa FM, Fernandes MH, Batistuzzo de Medeiros SR. 2020; Genotoxicity of root canal sealers: a literature review. Clin Oral Investig. 24:3347–62. DOI: 10.1007/s00784-020-03478-z. PMID: 32767107.
Article10. Tanomaru Filho M, Leonardo MR, Silva L, Utrilla LS. 1998; Effect of different root canal sealers on periapical repair of teeth with chronic periradicular periodontitis. lnt Endod J. 31:85–9. DOI: 10.1046/j.1365-2591.1998.00134.x. PMID: 9868933.
Article11. Fristad I, Molven O, Halse A. 2004; Nonsurgically retreated root filled teeth - radiographic findings after 20-27 years. lnt Endod J. 37:12–8. DOI: 10.1111/j.1365-2591.2004.00743.x. PMID: 14718052.12. Palma PJ, Ramos JC, Martins JB, Diogenes A, Figueiredo MH, Ferreira P, Viegas C, Santos JM. 2017; Histologic evaluation of regenerative endodontic procedures with the use of chitosan scaffolds in immature dog teeth with apical periodontitis. J Endod. 43:1279–87. DOI: 10.1016/j.joen.2017.03.005. PMID: 28577961.
Article13. Donnermeyer D, Bürklein S, Dammaschke T, Schäfer E. 2019; Endodontic sealers based on calcium silicates: a systematic review. Odontology. 107:421–36. DOI: 10.1007/s10266-018-0400-3. PMID: 30554288.
Article14. Sfeir G, Zogheib C, Patel S, Giraud T, Nagendrababu V, Bukiet F. 2021; Calcium silicate-based root canal sealers: A narrative review and clinical perspectives. Materials. 14:3965. DOI: 10.3390/ma14143965. PMID: 34300886. PMCID: PMC8306764.
Article15. Rodríguez-Lozano FJ, Collado-González M, Tomás-Catalá CJ, García-Bernal D, López S, Oñate-Sánchez RE, Moraleda JM, Murcia L. 2019; GuttaFlow Bioseal promotes spontaneous differentiation of human periodontal ligament stem cells into cementoblast-like cells. Dent Mater. 35:114–24. DOI: 10.1016/j.dental.2018.11.003. PMID: 30466731.
Article16. Kim M, Hayashi M, Yu B, Lee TK, Kim RH, Jo DW. 2022; Cytotoxicity and Genotoxicity of Epoxy Resin-Based Root Canal Sealers before and after Setting Procedures. Life (Basel). 12:847. DOI: 10.3390/life12060847. PMID: 35743878. PMCID: PMC9227444.
Article17. Silva Almeida LH, Moraes RR, Morgental RD, Pappen FG. 2017; Are Premixed Calcium Silicate-based Endodontic Sealers Comparable to Conventional Materials? A Systematic Review of In Vitro Studies. J Endod. 43:527–35. DOI: 10.1016/j.joen.2016.11.019. PMID: 28216270.18. Zoufan K, Jiang J, Komabayashi T, Wang YH, Safavi KE, Zhu Q. 2011; Cytotoxicity evaluation of Gutta flow and endo sequence BC sealers. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 112:657–61. DOI: 10.1016/j.tripleo.2011.03.050. PMID: 21778087.
Article19. Park MG, Kim IR, Kim HJ, Kwak SW, Kim HC. 2021; Physicochemical properties and cytocompatibility of newly developed calcium silicate-based sealers. Aust Endod J. 47:512–9. DOI: 10.1111/aej.12515. PMID: 33894082.
Article20. Koo J, Kwak SW, Kim HC. 2023; Differences in setting time of calcium silicate-based sealers under different test conditions. J Dent Sci. 18:1042–6. DOI: 10.1016/j.jds.2022.11.029. PMID: 37404604. PMCID: PMC10316423.
Article21. Schmidmaier G, Wildemann B, Melis B, Krummrey G, Einhorn TA, Haas NP, Raschke M. 2004; Development and characterization of a standard closed tibial fracture model in the rat. Eur J Trauma. 30:35–42. DOI: 10.1007/s00068-004-1322-z.
Article22. Claes L, Blakytny R, Göckelmann M, Schoen M, Ignatius A, Willie B. 2009; Early dynamization by reduced fixation stiffness does not improve fracture healing in a rat femoral osteotomy model. J Orthop Res. 27:22–7. DOI: 10.1002/jor.20712. PMID: 18634011.
Article23. Histing T, Garcia P, Holstein JH, Klein M, Matthys R, Nuetzi R, Steck R, Laschke MW, Wehner T, Bindl R, Recknagel S, Stuermer EK, Vollmar B, Wildemann B, Lienau J, Willie B, Peters A, Ignatius A, Pohlemann T, Claes L, Menger MD. 2011; Small animal bone healing models: standards, tips, and pitfalls results of a consensus meeting. Bone. 49:591–9. DOI: 10.1016/j.bone.2011.07.007. PMID: 21782988.
Article24. Bagi CM, Berryman E, Moalli MR. 2011; Comparative bone anatomy of commonly used laboratory animals: implications for drug discovery. Comp Med. 61:76–85.25. Moretton TR, Brown CE Jr, Legan JJ, Kafrawy AH. 2000; Tissue reactions after subcutaneous and intraosseous implantation of mineral trioxide aggregate and ethoxybenzoic acid cement. J Biomed Mater Res. 52:528–33. DOI: 10.1002/1097-4636(20001205)52:3<528::AID-JBM11>3.0.CO;2-9. PMID: 11007621.
Article26. Cintra LTA, de Moraes IG, Estrada BPF, Gomes-Filho JE, Bramante CM, Garcia RB, Bernardinelli N. 2006; Evaluation of the tissue response to MTA and MBPC: Microscopic analysis of implants in alveolar bone of rats. J Endod. 32:556–9. DOI: 10.1016/j.joen.2005.07.007. PMID: 16728250.
Article27. Assmann E, Böttcher DE, Hoppe CB, Grecca FS, Kopper PMP. 2015; Evaluation of bone tissue response to a sealer containing mineral trioxide aggregate. J Endod. 41:62–6. DOI: 10.1016/j.joen.2014.09.019. PMID: 25447498.
Article28. Almeida LH, Gomes APN, Gastmann AH, Pola NM, Moraes RR, Morgental RD, Cava SS, Felix AOC, Pappen FG. 2019; Bone tissue response to an MTA-based endodontic sealer, and the effect of the addition of calcium aluminate and silver particles. Int Endod J. 52:1446–56. DOI: 10.1111/iej.13135. PMID: 31034099.29. Figueiredo JA, Pesce HF, Gioso MA, Figueiredo MA. 2001; The histological effects of four endodontic sealers implanted in the oral mucosa: submucous injection versus implant in polyethylene tubes. Int Endod J. 34:377–85. DOI: 10.1046/j.1365-2591.2001.00407.x. PMID: 11482721.30. Trejo-Iriarte CG, Serrano-Bello J, Gutiérrez-Escalona R, Mercado-Marques C, García-Honduvilla N, Buján-Varela J, Medina LA. 2019; Evaluation of bone regeneration in a critical size cortical bone defect in rat mandible using microCT and histological analysis. Arch Oral Biol. 101:165–71. DOI: 10.1016/j.archoralbio.2019.01.010. PMID: 30951954.31. López-García S, Baek MH, Lozano A, García-Bernal D, Forner L, Llena C, Guerrero-Gironés J, Murcia L, Rodríguez-Lozano FJ. 2020; Cytocompatibility, bioactivity potential, and ion release of three premixed calcium silicate-based sealers. Clin Oral Investig. 24:1749–59. DOI: 10.1007/s00784-019-03036-2. PMID: 31399829.
Article32. Rodríguez-Lozano FJ, López-García S, García-Bernal D, Tomás-Catalá CJ, Santos JM, Llena C, Lozano A, Murcia L, Forner L. 2020; Chemical composition and bioactivity potential of the new Endosequence BC Sealer formulation HiFlow. Int Endod J. 53:1216–28. DOI: 10.1111/iej.13327. PMID: 32412113.33. Pinheiro LS, Iglesias JE, Boijink D, Mestieri LB, Kopper PMP, de Poli Figueiredo JA, Grecca FS. 2018; Cell viability and tissue reaction of NeoMTA Plus: an in vitro and in vivo study. J Endod. 44:1140–5. DOI: 10.1016/j.joen.2018.03.007. PMID: 29866406.34. Giacomino CM, Wealleans JA, Kuhn N, Diogenes A. 2019; Comparative biocompatibility and osteogenic potential of two bioceramic sealers. J Endod. 45:51–6. DOI: 10.1016/j.joen.2018.08.007. PMID: 30558798.35. Zamparini F, Prati C, Taddei P, Spinelli A, Di Foggia M, Gandolfi MG. 2022; Chemical-Physical Properties and Bioactivity of New Premixed Calcium Silicate-Bioceramic Root Canal Sealers. Int J Mol Sci. 23:13914. DOI: 10.3390/ijms232213914. PMID: 36430393. PMCID: PMC9692705.
Article36. Santos JM, Coelho CM, Sequeira DB, Marques JA, Pereira JF, Sousa V, Palma PJ, Santos AC. 2021; Subcutaneous Implantation Assessment of New Calcium-Silicate Based Sealer for Warm Obturation. Biomedicines. 9:24. DOI: 10.3390/biomedicines9010024. PMID: 33401424. PMCID: PMC7824331.37. Wada T, Nakashima T, Hiroshi N, Penninger JM. 2006; RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med. 12:17–25. DOI: 10.1016/j.molmed.2005.11.007. PMID: 16356770.38. Rufo A, Del Fattore A, Capulli M, Carvello F, De Pasquale L, Ferrari S, Pierroz D, Morandi L, De Simone M, Rucci N, Bertini E, Bianchi ML, De Benedetti F, Teti A. 2011; Mechanisms inducing low bone density in Duchenne muscular dystrophy in mice and humans. J Bone Miner Res. 26:1891–903. DOI: 10.1002/jbmr.410. PMID: 21509823. PMCID: PMC3150693.39. Sanz JL, López-García S, Rodríguez-Lozano FJ, Melo M, Lozano A, Llena C, Forner L. 2022; Cytocompatibility and bioactive potential of AH Plus Bioceramic Sealer: An in vitro study. Int Endod J. 55:1066–80. DOI: 10.1111/iej.13805. PMID: 35950780. PMCID: PMC9541143.40. Kuboki Y, Takita H, Kobayashi D, Tsuruga E, Inoue M, Murata M, Nagai N, Dohi Y, Ohgushi H. 1998; BMP-induced osteogenesis on the surface of hydroxyapatite with geometrically feasible and nonfeasible structures: topology of osteogenesis. J Biomed Mater Res. 39:190–9. DOI: 10.1002/(SICI)1097-4636(199802)39:2<190::AID-JBM4>3.0.CO;2-K.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nanoleakage of apical sealing using a calcium silicate-based sealer according to canal drying methods
- Calcium silicate-based root canal sealers: a literature review
- Comparison of sealing ability of different obturation techniques in type II root canals
- Efficacy of retreatment NiTi files for root canals filled with calcium silicate-based sealer
- A scientometric, bibliometric, and thematic map analysis of hydraulic calcium silicate root canal sealers