Diabetes Metab J.
2024 Mar;48(2):302-311. 10.4093/dmj.2022.0255.
Impact of Hyperglycemia on Complication and Mortality after Transarterial Chemoembolization for Hepatocellular Carcinoma
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
- 2Division of Endocrinology and Metabolism, Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- 3Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- 4Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea
- KMID: 2553600
- DOI: http://doi.org/10.4093/dmj.2022.0255
Abstract
- Background
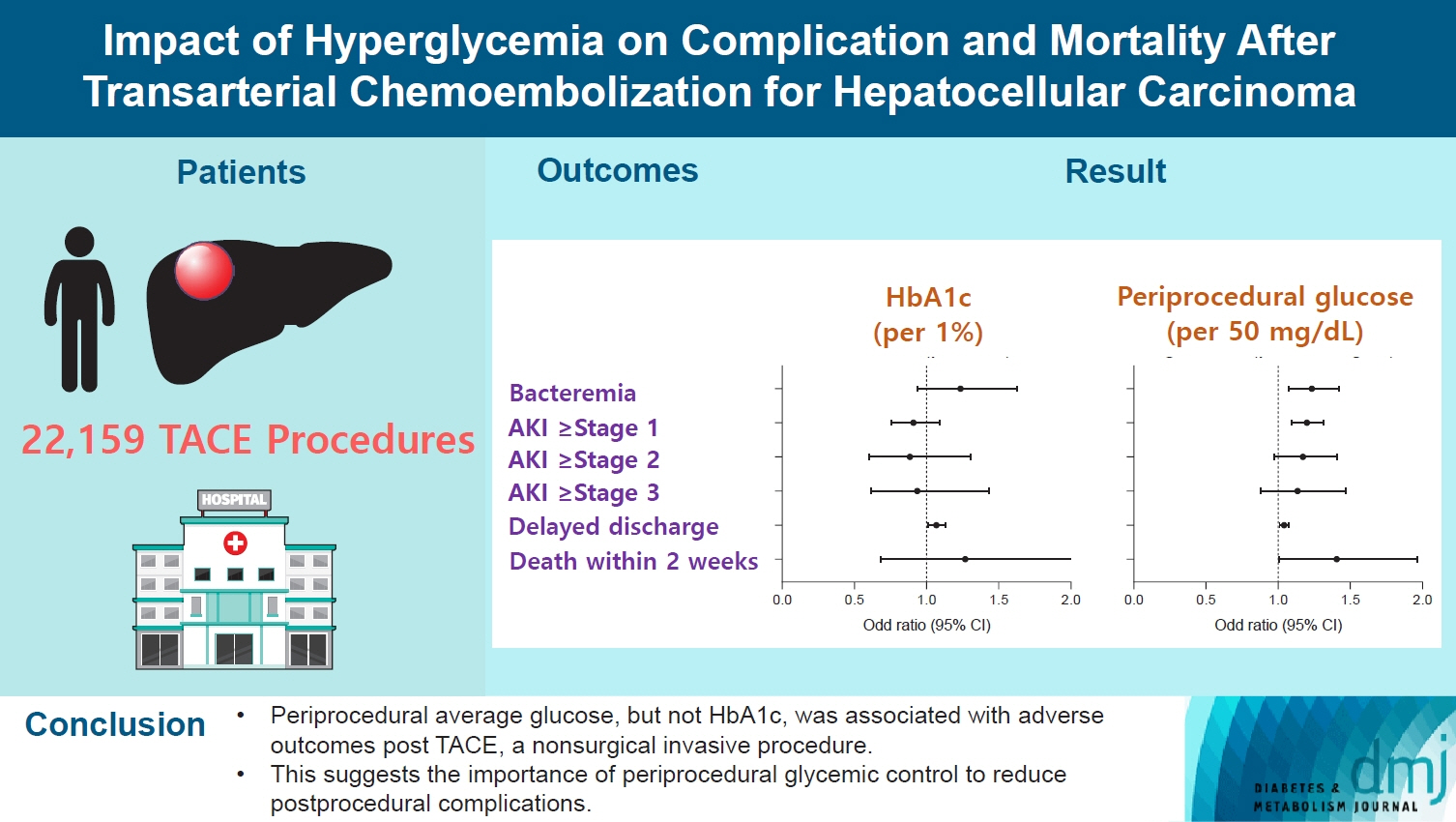
Current guidelines regarding periprocedural glycemic control to prevent complications after nonsurgical invasive procedures are insufficient. Transarterial chemoembolization (TACE) is a widely used treatment for unresectable hepatocellular carcinoma. We aimed to investigate the association between diabetes mellitus (DM) per se and the degree of hyperglycemia with postprocedural complications after TACE.
Methods
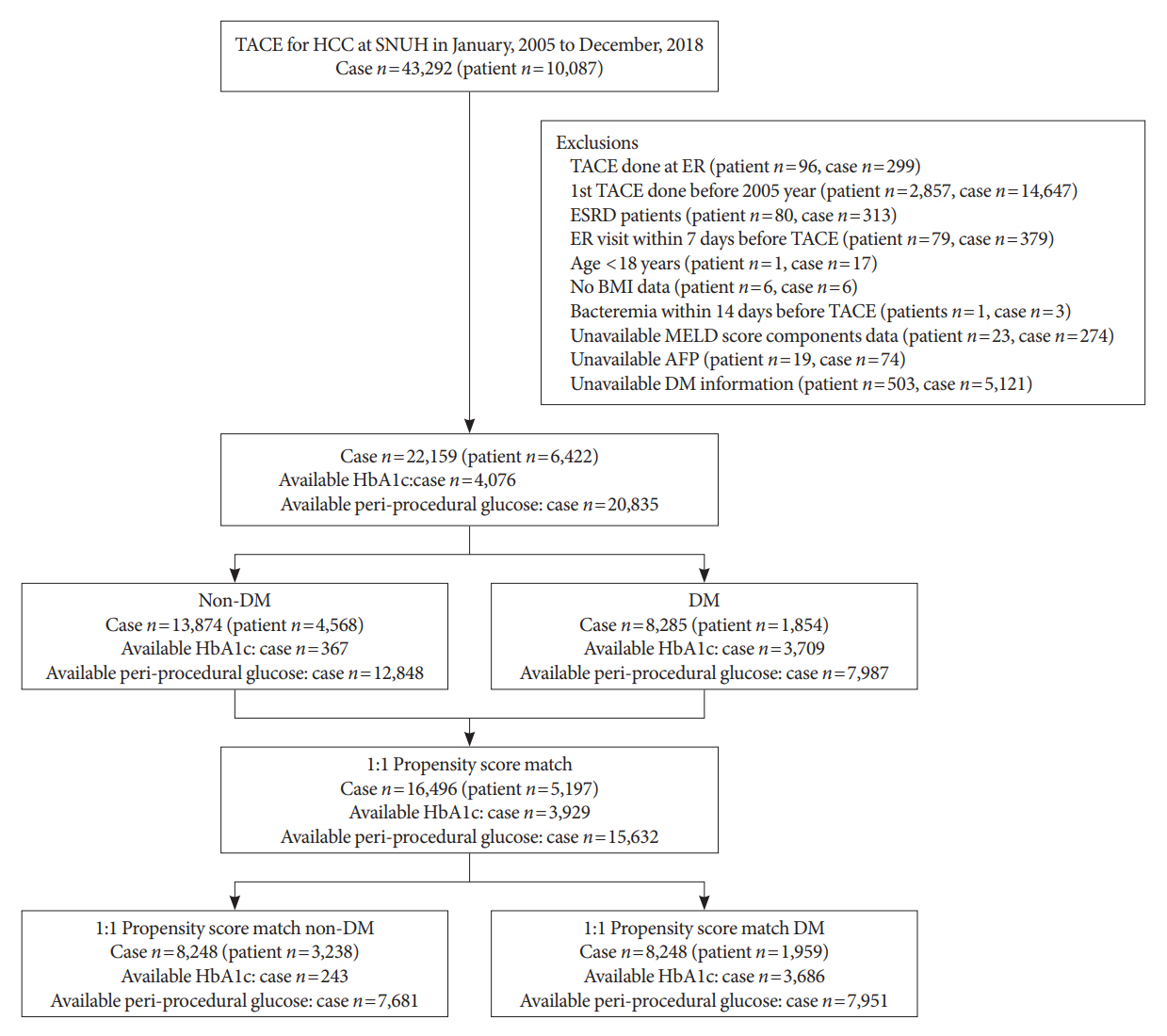
A total of 22,159 TACE procedures performed at Seoul National University Hospital from 2005 to 2018 were retrospectively analyzed. The associations between DM, preprocedural glycosylated hemoglobin (HbA1c), and periprocedural average glucose with postprocedural adverse outcomes were evaluated. The primary outcome was occurrence of postprocedural bacteremia. Secondary outcomes were acute kidney injury (AKI), delayed discharge and death within 14 days. Periprocedural glucose was averaged over 3 days: the day of, before, and after the TACE procedures. Propensity score matching was applied for procedures between patients with or without DM.
Results
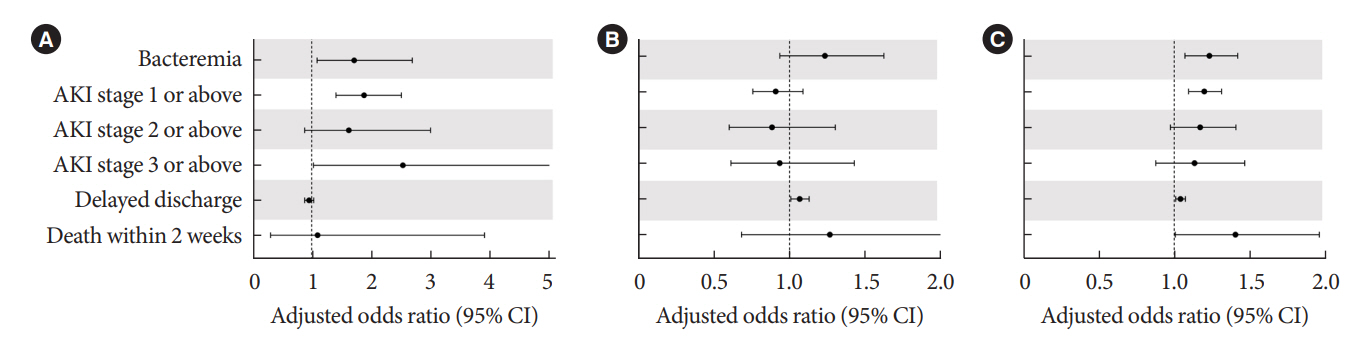
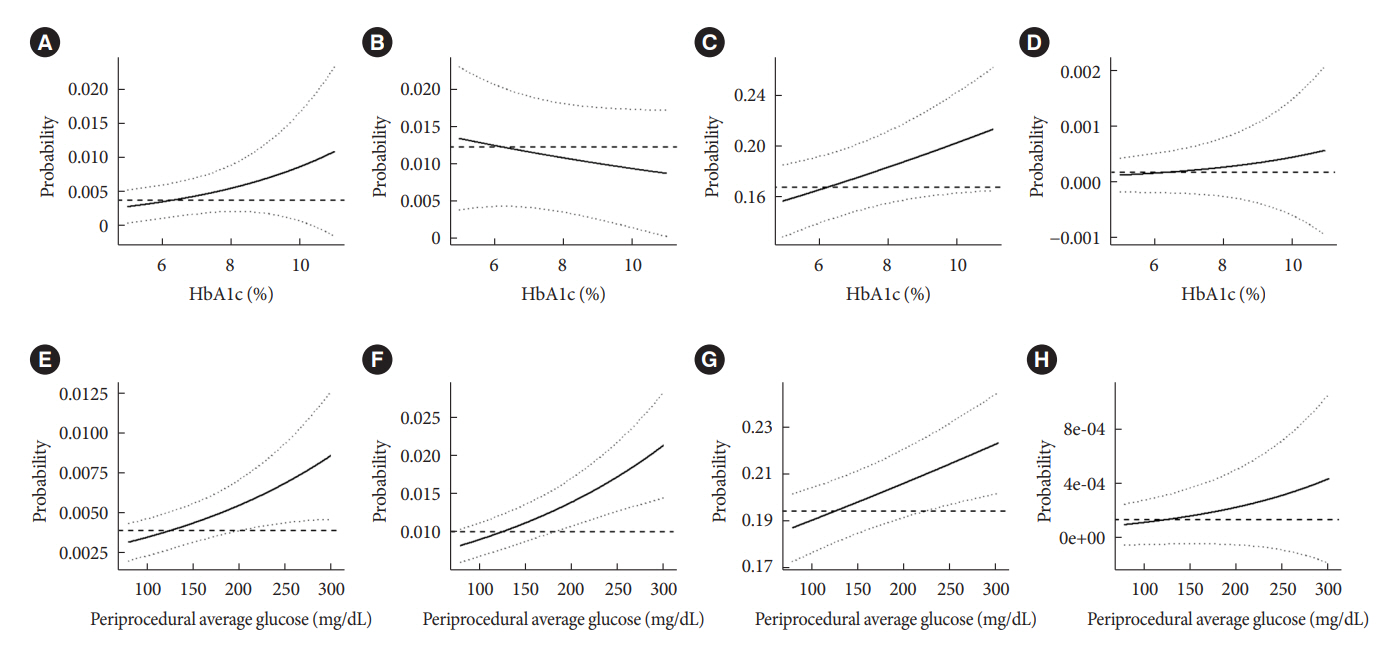
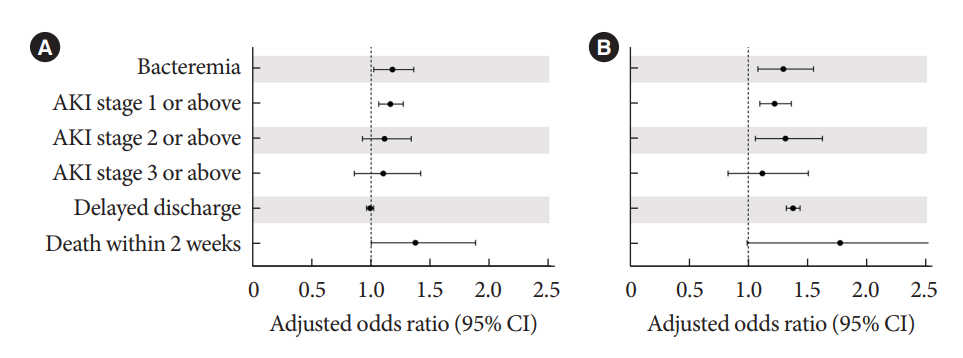
Periprocedural average glucose was significantly associated with bacteremia (adjusted odds ratio per 50 mg/dL of glucose, 1.233; 95% confidence interval, 1.071 to 1.420; P=0.004), AKI, delayed discharge, and death within 14 days. DM per se was only associated with bacteremia and AKI. Preprocedural HbA1c was associated with delayed discharge. Average glucose levels above 202 and 181 mg/dL were associated with a significantly higher risk of bacteremia and AKI, respectively, than glucose levels of 126 mg/dL or lower.
Conclusion
Periprocedural average glucose, but not HbA1c, was associated with adverse outcomes after TACE, which is a nonsurgical invasive procedure. This suggests the importance of periprocedural glycemic control to reduce postprocedural complications.
Keyword
Figure
Reference
-
1. Frisch A, Chandra P, Smiley D, Peng L, Rizzo M, Gatcliffe C, et al. Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care. 2010; 33:1783–8.
Article2. Kwon S, Thompson R, Dellinger P, Yanez D, Farrohki E, Flum D. Importance of perioperative glycemic control in general surgery: a report from the Surgical Care and Outcomes Assessment Program. Ann Surg. 2013; 257:8–14.
Article3. Thourani VH, Weintraub WS, Stein B, Gebhart SS, Craver JM, Jones EL, et al. Influence of diabetes mellitus on early and late outcome after coronary artery bypass grafting. Ann Thorac Surg. 1999; 67:1045–52.
Article4. van den Boom W, Schroeder RA, Manning MW, Setji TL, Fiestan GO, Dunson DB. Effect of A1C and glucose on postoperative mortality in noncardiac and cardiac surgeries. Diabetes Care. 2018; 41:782–8.
Article5. American Diabetes Association. 15. Diabetes care in the hospital: standards of medical care in diabetes-2021. Diabetes Care. 2021; 44(Suppl 1):S211–20.6. Joshi GP, Chung F, Vann MA, Ahmad S, Gan TJ, Goulson DT, et al. Society for Ambulatory Anesthesia consensus statement on perioperative blood glucose management in diabetic patients undergoing ambulatory surgery. Anesth Analg. 2010; 111:1378–87.
Article7. Umpierrez GE, Hellman R, Korytkowski MT, Kosiborod M, Maynard GA, Montori VM, et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012; 97:16–38.
Article8. Hoole SP, Bambrough P. Recent advances in percutaneous coronary intervention. Heart. 2020; 106:1380–6.
Article9. Lindsay J, Sharma AK, Canos D, Nandalur M, Pinnow E, Apple S, et al. Preprocedure hyperglycemia is more strongly associated with restenosis in diabetic patients after percutaneous coronary intervention than is hemoglobin A1C. Cardiovasc Revasc Med. 2007; 8:15–20.
Article10. Wang Y, Liu K, Xie X, Song B. Contrast-associated acute kidney injury: an update of risk factors, risk factor scores, and preventive measures. Clin Imaging. 2021; 69:354–62.
Article11. Ritsinger V, Malmberg K, Martensson A, Ryden L, Wedel H, Norhammar A. Intensified insulin-based glycaemic control after myocardial infarction: mortality during 20 year follow-up of the randomised Diabetes Mellitus Insulin Glucose Infusion in Acute Myocardial Infarction (DIGAMI 1) trial. Lancet Diabetes Endocrinol. 2014; 2:627–33.
Article12. European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer. EASLEORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012; 56:908–43.13. Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatology. 2016; 64:106–16.
Article14. Tolman KG, Fonseca V, Dalpiaz A, Tan MH. Spectrum of liver disease in type 2 diabetes and management of patients with diabetes and liver disease. Diabetes Care. 2007; 30:734–43.
Article15. Zein NN, Abdulkarim AS, Wiesner RH, Egan KS, Persing DH. Prevalence of diabetes mellitus in patients with end-stage liver cirrhosis due to hepatitis C, alcohol, or cholestatic disease. J Hepatol. 2000; 32:209–17.
Article16. Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007; 11:R31.
Article17. Kotagal M, Symons RG, Hirsch IB, Umpierrez GE, Dellinger EP, Farrokhi ET, et al. Perioperative hyperglycemia and risk of adverse events among patients with and without diabetes. Ann Surg. 2015; 261:97–103.
Article18. Gandhi GY, Murad MH, Flynn DN, Erwin PJ, Cavalcante AB, Bay Nielsen H, et al. Effect of perioperative insulin infusion on surgical morbidity and mortality: systematic review and meta-analysis of randomized trials. Mayo Clin Proc. 2008; 83:418–30.
Article19. Pietrosi G, Miraglia R, Luca A, Vizzini GB, Fili’ D, Riccardo V, et al. Arterial chemoembolization/embolization and early complications after hepatocellular carcinoma treatment: a safe standardized protocol in selected patients with child class A and B cirrhosis. J Vasc Interv Radiol. 2009; 20:896–902.
Article20. Chung JW, Park JH, Han JK, Choi BI, Han MC, Lee HS, et al. Hepatic tumors: predisposing factors for complications of transcatheter oily chemoembolization. Radiology. 1996; 198:33–40.
Article21. Silver SA, Shah PM, Chertow GM, Harel S, Wald R, Harel Z. Risk prediction models for contrast induced nephropathy: systematic review. BMJ. 2015; 351:h4395.
Article22. Heyman SN, Rosenberger C, Rosen S, Khamaisi M. Why is diabetes mellitus a risk factor for contrast-induced nephropathy? Biomed Res Int. 2013; 2013:123589.
Article23. Carey IM, Critchley JA, DeWilde S, Harris T, Hosking FJ, Cook DG. Risk of infection in type 1 and type 2 diabetes compared with the general population: a matched cohort study. Diabetes Care. 2018; 41:513–21.
Article24. Critchley JA, Carey IM, Harris T, DeWilde S, Hosking FJ, Cook DG. Glycemic control and risk of infections among people with type 1 or type 2 diabetes in a large primary care cohort study. Diabetes Care. 2018; 41:2127–35.
Article25. Peleg AY, Weerarathna T, McCarthy JS, Davis TM. Common infections in diabetes: pathogenesis, management and relationship to glycaemic control. Diabetes Metab Res Rev. 2007; 23:3–13.
Article26. Schuetz P, Castro P, Shapiro NI. Diabetes and sepsis: preclinical findings and clinical relevance. Diabetes Care. 2011; 34:771–8.
Article27. Alexiewicz JM, Kumar D, Smogorzewski M, Klin M, Massry SG. Polymorphonuclear leukocytes in non-insulin-dependent diabetes mellitus: abnormalities in metabolism and function. Ann Intern Med. 1995; 123:919–24.
Article28. Rubinstein R, Genaro AM, Motta A, Cremaschi G, Wald MR. Impaired immune responses in streptozotocin-induced type I diabetes in mice: involvement of high glucose. Clin Exp Immunol. 2008; 154:235–46.
Article29. Nadelson J, Satapathy SK, Nair S. Glycated hemoglobin levels in patients with decompensated cirrhosis. Int J Endocrinol. 2016; 2016:8390210.
Article30. Sehrawat T, Jindal A, Kohli P, Thour A, Kaur J, Sachdev A, et al. Utility and limitations of glycated hemoglobin (HbA1c) in patients with liver cirrhosis as compared with oral glucose tolerance test for diagnosis of diabetes. Diabetes Ther. 2018; 9:243–51.
Article31. Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of inhospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002; 87:978–82.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Complications Related to Transarterial Treatment of Hepatocellular Carcinoma: A Comprehensive Review
- Efficacy of Hepatic Arterial Infusion Chemotherapy and Radiofrequency Ablation against Hepatocellular Carcinoma Refractory to Transarterial Chemoembolization and Vascular Variation: A Case Study
- Transarterial Chemoembolization in Patients with Hepatocellular Carcinoma and Extrahepatic Metastasis
- Which treatment modality should we choose for advanced hepatocellular carcinoma?
- Comparison of surgical resection versus transarterial chemoembolization with additional radiation therapy in patients with hepatocellular carcinoma with portal vein invasion