J Pathol Transl Med.
2024 Mar;58(2):59-71. 10.4132/jptm.2024.01.04.
Clinicopathological implications of immunohistochemical expression of TBX21, CXCR3, GATA3, CCR4, and TCF1 in nodal follicular helper T-cell lymphoma and peripheral T-cell lymphoma, not otherwise specified
- Affiliations
-
- 1Department of Pathology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 2Department of Pathology, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul, Korea
- 3Department of Pathology, Korea University Guro Hospital, Seoul, Korea
- 4Seoul National University Cancer Research Institute, Seoul, Korea
- KMID: 2553473
- DOI: http://doi.org/10.4132/jptm.2024.01.04
Abstract
- Background
The classification of nodal peripheral T-cell lymphoma (PTCL) has evolved according to histology, cell-of-origin, and genetic alterations. However, the comprehensive expression pattern of follicular helper T-cell (Tfh) markers, T-cell factor-1 (TCF1), and Th1- and Th2-like molecules in nodal PTCL is unclear.
Methods
Eighty-two cases of nodal PTCL were classified into 53 angioimmunoblastic T-cell lymphomas (AITLs)/nodal T-follicular helper cell lymphoma (nTFHL)-AI, 18 PTCLs-Tfh/nTFHL–not otherwise specified (NOS), and 11 PTCLs-NOS according to the revised 4th/5th World Health Organization classifications. Immunohistochemistry for TCF1, TBX21, CXCR3, GATA3, and CCR4 was performed.
Results
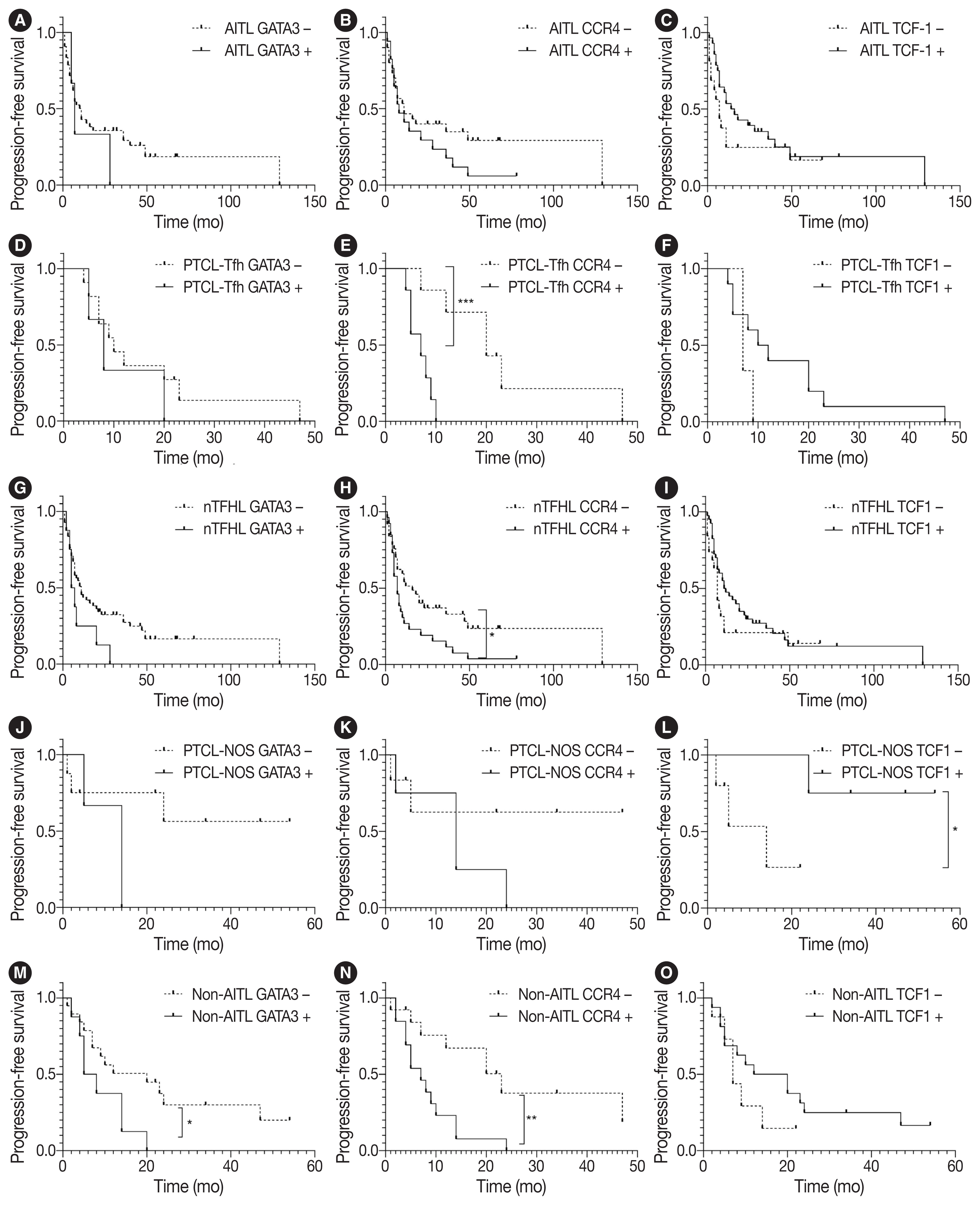
TCF1 was highly expressed in up to 68% of patients with nTFHL but also in 44% of patients with PTCL-NOS (p > .05). CXCR3 expression was higher in AITLs than in non-AITLs (p = .035), whereas GATA3 expression was higher in non-AITL than in AITL (p = .007) and in PTCL-Tfh compared to AITL (p = .010). Of the cases, 70% of AITL, 44% of PTCLTfh/ nTFHL-NOS, and 36% of PTCL-NOS were subclassified as the TBX21 subtype; and 15% of AITL, 38% of PTCL-Tfh/nTFHL-NOS, and 36% of PTCL-NOS were subclassified as the GATA3 subtype. The others were an unclassified subtype. CCR4 expression was associated with poor progression-free survival (PFS) in patients with PTCL-Tfh (p < .001) and nTFHL (p = .023). The GATA3 subtype showed poor overall survival in PTCL-NOS compared to TBX21 (p = .046) and tended to be associated with poor PFS in patients with non-AITL (p = .054).
Conclusions
The TBX21 subtype was more prevalent than the GATA3 subtype in AITL. The GATA3 subtype was associated with poor prognosis in patients with non-AITL and PTCL-NOS.
Keyword
Figure
Reference
-
References
1. Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. Revised 4th ed. Geneva: World Health Organization;2017.2. Alaggio R, Amador C, Anagnostopoulos I, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia. 2022; 36:1720–48.3. Campo E, Jaffe ES, Cook JR, et al. The International Consensus Classification of Mature Lymphoid Neoplasms: a report from the Clinical Advisory Committee. Blood. 2022; 140:1229–53.4. Vallois D, Dobay MP, Morin RD, et al. Activating mutations in genes related to TCR signaling in angioimmunoblastic and other follicular helper T-cell-derived lymphomas. Blood. 2016; 128:1490–502.5. Palomero T, Couronne L, Khiabanian H, et al. Recurrent mutations in epigenetic regulators, RHOA and FYN kinase in peripheral T cell lymphomas. Nat Genet. 2014; 46:166–70.6. Lemonnier F, Couronne L, Parrens M, et al. Recurrent TET2 mutations in peripheral T-cell lymphomas correlate with TFH-like features and adverse clinical parameters. Blood. 2012; 120:1466–9.7. Yoo HY, Sung MK, Lee SH, et al. A recurrent inactivating mutation in RHOA GTPase in angioimmunoblastic T cell lymphoma. Nat Genet. 2014; 46:371–5.8. Sakata-Yanagimoto M, Enami T, Yoshida K, et al. Somatic RHOA mutation in angioimmunoblastic T cell lymphoma. Nat Genet. 2014; 46:171–5.9. Dobay MP, Lemonnier F, Missiaglia E, et al. Integrative clinicopathological and molecular analyses of angioimmunoblastic T-cell lymphoma and other nodal lymphomas of follicular helper T-cell origin. Haematologica. 2017; 102:e148–51.10. Sandell RF, Boddicker RL, Feldman AL. Genetic landscape and classification of peripheral T cell lymphomas. Curr Oncol Rep. 2017; 19:28.11. Ondrejka SL, Amador C, Climent F, et al. Follicular helper T-cell lymphomas: disease spectrum, relationship with clonal hematopoiesis, and mimics.. A report of the 2022 EA4HP/SH lymphoma workshop. Virchows Arch. 2023; 483:349–65.12. Vega F, Medeiros LJ. A suggested immunohistochemical algorithm for the classification of T-cell lymphomas involving lymph nodes. Hum Pathol. 2020; 102:104–16.13. Basha BM, Bryant SC, Rech KL, et al. Application of a 5 marker panel to the routine diagnosis of peripheral T-cell lymphoma with T-follicular helper phenotype. Am J Surg Pathol. 2019; 43:1282–90.14. Kim C, Jin J, Weyand CM, Goronzy JJ. The transcription factor TCF1 in T cell differentiation and aging. Int J Mol Sci. 2020; 21:6497.15. Qiu H, Wu H, Chan V, Lau CS, Lu Q. Transcriptional and epigenetic regulation of follicular T-helper cells and their role in autoimmunity. Autoimmunity. 2017; 50:71–81.16. Wang T, Feldman AL, Wada DA, et al. GATA-3 expression identifies a high-risk subset of PTCL, NOS with distinct molecular and clinical features. Blood. 2014; 123:3007–15.17. Iqbal J, Weisenburger DD, Greiner TC, et al. Molecular signatures to improve diagnosis in peripheral T-cell lymphoma and prognostication in angioimmunoblastic T-cell lymphoma. Blood. 2010; 115:1026–36.18. Heavican TB, Bouska A, Yu J, et al. Genetic drivers of oncogenic pathways in molecular subgroups of peripheral T-cell lymphoma. Blood. 2019; 133:1664–76.19. Iqbal J, Wright G, Wang C, et al. Gene expression signatures delineate biological and prognostic subgroups in peripheral T-cell lymphoma. Blood. 2014; 123:2915–23.20. Amador C, Greiner TC, Heavican TB, et al. Reproducing the molecular subclassification of peripheral T-cell lymphoma-NOS by immunohistochemistry. Blood. 2019; 134:2159–70.21. Ioannidou K, Ndiaye DR, Noto A, et al. In situ characterization of follicular helper CD4 T cells using multiplexed imaging. Front Immunol. 2020; 11:607626.22. Wang P, Wang Y, Xie L, et al. The transcription factor T-bet is required for optimal type I follicular helper T cell maintenance during acute viral infection. Front Immunol. 2019; 10:606.23. Sheikh AA, Groom JR. Transcription tipping points for T follicular helper cell and T-helper 1 cell fate commitment. Cell Mol Immunol. 2021; 18:528–38.24. Schmitt N, Bentebibel SE, Ueno H. Phenotype and functions of memory Tfh cells in human blood. Trends Immunol. 2014; 35:436–42.25. Paik JH, Koh J, Han B, et al. Distinct and overlapping features of nodal peripheral T-cell lymphomas exhibiting a follicular helper T-cell phenotype: a multicenter study emphasizing the clinicopathological significance of follicular helper T-cell marker expression. Hum Pathol. 2023; 131:47–60.26. Kim S, Kwon D, Koh J, et al. Clinicopathological features of programmed cell death-1 and programmed cell death-ligand-1 expression in the tumor cells and tumor microenvironment of angioimmunoblastic T cell lymphoma and peripheral T cell lymphoma not otherwise specified. Virchows Arch. 2020; 477:131–42.27. Vega F, Amador C, Chadburn A, et al. Genetic profiling and biomarkers in peripheral T-cell lymphomas: current role in the diagnostic work-up. Mod Pathol. 2022; 35:306–18.28. Ghione P, Faruque P, Mehta-Shah N, et al. T follicular helper phenotype predicts response to histone deacetylase inhibitors in relapsed/refractory peripheral T-cell lymphoma. Blood Adv. 2020; 4:4640–7.29. Drieux F, Lemonnier F, Gaulard P. How molecular advances may improve the diagnosis and management of PTCL patients. Front Oncol. 2023; 13:1202964.30. Yu S, Zhou X, Steinke FC, et al. The TCF-1 and LEF-1 transcription factors have cooperative and opposing roles in T cell development and malignancy. Immunity. 2012; 37:813–26.31. Tiemessen MM, Baert MR, Schonewille T, et al. The nuclear effector of Wnt-signaling, Tcf1, functions as a T-cell-specific tumor suppressor for development of lymphomas. PLoS Biol. 2012; 10:e1001430.32. Arnovitz S, Mathur P, Tracy M, et al. Tcf-1 promotes genomic instability and T cell transformation in response to aberrant beta-catenin activation. Proc Natl Acad Sci U S A. 2022; 119:e2201493119.33. Dorfman DM, Greisman HA, Shahsafaei A. Loss of expression of the WNT/beta-catenin-signaling pathway transcription factors lymphoid enhancer factor-1 (LEF-1) and T cell factor-1 (TCF-1) in a subset of peripheral T cell lymphomas. Am J Pathol. 2003; 162:1539–44.34. Yoon SE, Cho J, Kim YJ, et al. Comprehensive analysis of clinical, pathological, and genomic characteristics of follicular helper T-cell derived lymphomas. Exp Hematol Oncol. 2021; 10:33.35. Geng X, Wang C, Gao X, et al. GATA-3 is a proto-oncogene in T-cell lymphoproliferative neoplasms. Blood Cancer J. 2022; 12:149.36. Maura F, Dodero A, Carniti C, et al. CDKN2A deletion is a frequent event associated with poor outcome in patients with peripheral T-cell lymphoma not otherwise specified (PTCL-NOS). Haematologica. 2021; 106:2918–26.37. Ishida T, Iida S, Akatsuka Y, et al. The CC chemokine receptor 4 as a novel specific molecular target for immunotherapy in adult T-Cell leukemia/lymphoma. Clin Cancer Res. 2004; 10:7529–39.38. Ishida T, Inagaki H, Utsunomiya A, et al. CXC chemokine receptor 3 and CC chemokine receptor 4 expression in T-cell and NK-cell lymphomas with special reference to clinicopathological significance for peripheral T-cell lymphoma, unspecified. Clin Cancer Res. 2004; 10:5494–500.39. Asano N, Suzuki R, Ohshima K, et al. Linkage of expression of chemokine receptors (CXCR3 and CCR4) and cytotoxic molecules in peripheral T cell lymphoma, not otherwise specified and ALK-negative anaplastic large cell lymphoma. Int J Hematol. 2010; 91:426–35.40. Campbell JJ, Clark RA, Watanabe R, Kupper TS. Sezary syndrome and mycosis fungoides arise from distinct T-cell subsets: a biologic rationale for their distinct clinical behaviors. Blood. 2010; 116:767–71.41. Ishida T, Utsunomiya A, Iida S, et al. Clinical significance of CCR4 expression in adult T-cell leukemia/lymphoma: its close association with skin involvement and unfavorable outcome. Clin Cancer Res. 2003; 9:3625–34.42. Ureshino H, Kamachi K, Kimura S. Mogamulizumab for the treatment of adult T-cell leukemia/lymphoma. Clin Lymphoma Myeloma Leuk. 2019; 19:326–31.43. Kasamon YL, Chen H, de Claro RA, et al. FDA Approval summary: mogamulizumab-kpkc for mycosis fungoides and Sezary syndrome. Clin Cancer Res. 2019; 25:7275–80.44. Ogura M, Ishida T, Hatake K, et al. Multicenter phase II study of mogamulizumab (KW-0761), a defucosylated anti-cc chemokine receptor 4 antibody, in patients with relapsed peripheral T-cell lymphoma and cutaneous T-cell lymphoma. J Clin Oncol. 2014; 32:1157–63.45. Ngu HS, Savage KJ. Past, present and future therapeutic approaches in nodal peripheral T-cell lymphomas. Haematologica. 2023; 108:3211–26.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Detection of bcl-2/IgH Gene Rearrangement and Expression of c-myc and p53 Oncoprotein in B-cell Lymphoma
- A case of nodal marginal zone B-cell lymphoma of the lower eyelid
- A Case of Primary Cutaneous Marginal Zone B-cell Lymphoma
- Selective addition of CXCR3+CCR4-CD4+ Th1 cells enhances generation of cytotoxic T cells by dendritic cells in vitro
- A Case of Recurred Follicular Lymphoma in Sublingual Gland after Complete Remission