J Korean Med Sci.
2024 Feb;39(6):e52. 10.3346/jkms.2024.39.e52.
Determining and Comparing the RealWorld Effectiveness of Molnupiravir and Nirmatrelvir-Ritonavir in Patients Hospitalized With COVID-19
- Affiliations
-
- 1Division of Infectious Diseases, Department of Internal Medicine, Incheon Medical Center, Incheon, Korea
- 2Division of Metabolism and Endocrinology, Department of Internal Medicine, Incheon Medical Center, Incheon, Korea
- KMID: 2553304
- DOI: http://doi.org/10.3346/jkms.2024.39.e52
Abstract
- Background
Current guidelines recommend using nirmatrelvir-ritonavir for coronavirus disease 2019 (COVID-19) treatment, but its potential drug interactions and contraindications limit its applicability in certain categories of patients. The aim of the study was to evaluate the real-world effectiveness of molnupiravir and nirmatrelvir-ritonavir in managing COVID-19 among hospitalized patients.
Methods
We conducted a retrospective cohort study among hospitalized COVID-19 patients who received molnupiravir or nirmatrelvir-ritonavir and did not require baseline supplemental oxygen from February 2022 to January 2023. We compared the effectiveness of molnupiravir and nirmatrelvir-ritonavir with a focus on disease progression.
Results
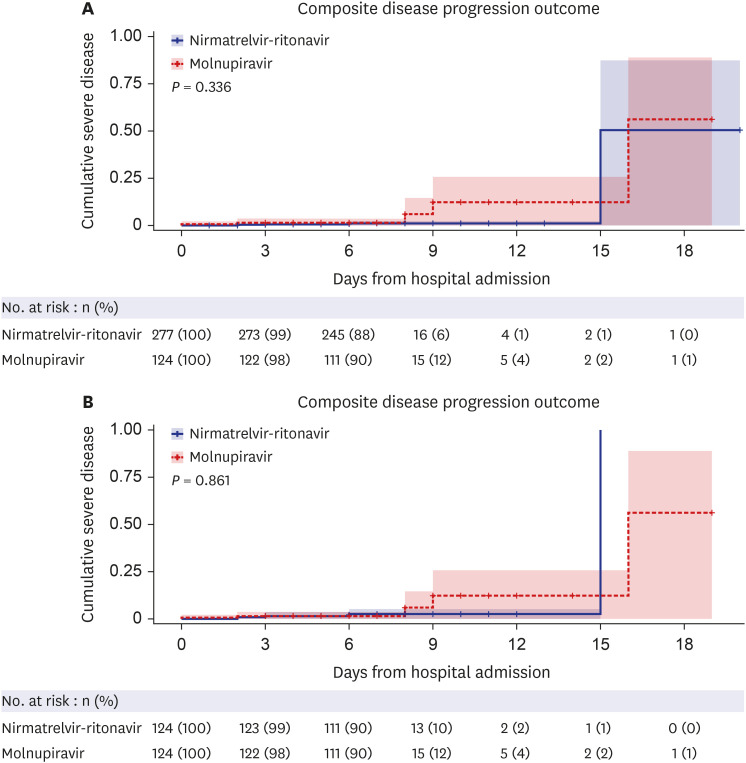
The study included 401 high-risk, hospitalized adult COVID-19 patients who received molnupiravir or nirmatrelvir-ritonavir. No significant difference was found in disease progression, the composite outcome of disease progression (4.0% vs. 1.4%, P = 0.782), and O2 supplementation via nasal prong (21.8% vs. 14.8%, P = 0.115) between the patients treated with molnupiravir and those treated with nirmatrelvir-ritonavir. This finding was similar after 1:1 propensity-score matching. In the multivariate analysis, molnupiravir treatment was not significantly associated with progression to severe disease.
Conclusion
In conclusion, our findings suggest that similar to nirmatrelvir-ritonavir, molnupiravir has a distinct potential role in COVID-19 treatment, transcending its current perceived status as only a secondary option.
Keyword
Figure
Reference
-
1. U.S. Food and Drug Administration. FDA approves first oral antiviral treatment for COVID-19 in adults. Updated 2023. Accessed July 12, 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-first-oral-antiviral-treatment-covid-19-adults .2. National Institutes of Health. Molnupiravir. COVID-19 treatment guidelines. Updated 2023. Accessed July 12, 2023. https://www.covid19treatmentguidelines.nih.gov/therapies/antivirals-including-antibody-products/molnupiravir/?utm_source=site&utm_medium=home&utm_campaign=highlights .3. Butler CC, Hobbs FDR, Gbinigie OA, Rahman NM, Hayward G, Richards DB, et al. Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial. Lancet. 2023; 401(10373):281–293. PMID: 36566761.4. Wong CK, Au IC, Lau KT, Lau EH, Cowling BJ, Leung GM. Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong’s omicron BA.2 wave: a retrospective cohort study. Lancet Infect Dis. 2022; 22(12):1681–1693. PMID: 36029795.5. Wong CK, Au IC, Lau KT, Lau EH, Cowling BJ, Leung GM. Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study. Lancet. 2022; 400(10359):1213–1222. PMID: 36216007.6. Wan EYF, Yan VKC, Mok AHY, Wang B, Xu W, Cheng FWT, et al. Effectiveness of molnupiravir and nirmatrelvir-ritonavir in hospitalized patients with COVID-19 : a target trial emulation study. Ann Intern Med. 2023; 176(4):505–514. PMID: 36913693.7. Park JJ, Kim H, Kim YK, Lee SS, Jung E, Lee JS, et al. Effectiveness and adverse events of nirmatrelvir/ritonavir versus molnupiravir for COVID-19 in outpatient setting: multicenter prospective observational study. J Korean Med Sci. 2023; 38(42):e347. PMID: 37904658.8. Ryu B, Shin E, Kim NY, Kim DH, Lee H, Kim A, et al. Severity of COVID-19 associated with SARS-CoV-2 variants circulating in the Republic of Korea. Public Health Weekly Report. 2022; 15(47):2873–2895.9. Korea Disease Control and Prevention Agency. COVID-19 treatment guidance. Accessed July 13, 2023. https://ncv.kdca.go.kr/pot/www/CVID19/TRTMNT/MEDCN.jsp?seq=TAB8_21 .10. Sung HK, Kim JY, Heo J, Seo H, Jang YS, Kim H, et al. Clinical course and outcomes of 3,060 patients with coronavirus disease 2019 in Korea, January-May 2020. J Korean Med Sci. 2020; 35(30):e280. PMID: 32743995.11. Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021; 385(19):1737–1749. PMID: 34554658.12. Arribas JR, Bhagani S, Lobo SM, Khaertynova I, Mateu L, Fishchuk R, et al. Randomized trial of molnupiravir or placebo in patients hospitalized with Covid-19. NEJM Evid. 2022; 1(2):EVIDoa2100044. PMID: 38319178.13. Johnson MG, Puenpatom A, Moncada PA, Burgess L, Duke ER, Ohmagari N, et al. Effect of molnupiravir on biomarkers, respiratory interventions, and medical services in COVID-19: a randomized, placebo-controlled trial. Ann Intern Med. 2022; 175(8):1126–1134. PMID: 35667065.14. Infectious Diseases Society of America. IDSA guidelines on the treatment and management of patients with COVID-19. Updated 2023. Accessed July 12, 2023. https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/ .15. National Institutes of Health, COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. Accessed July 12, 2023. https://www.covid19treatmentguidelines.nih.gov .16. World Health Organization. Therapeutics and COVID-19: living guideline. Updated 2023. Accessed July 12, 2023. https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2023.1 .17. Tiseo G, Barbieri C, Galfo V, Occhineri S, Matucci T, Almerigogna F, et al. Efficacy and safety of nirmatrelvir/ritonavir, molnupiravir, and remdesivir in a real-world cohort of outpatients with COVID-19 at high risk of progression: the PISA Outpatient Clinic Experience. Infect Dis Ther. 2023; 12(1):257–271. PMID: 36441485.18. Hoertel N, Boulware DR, Sánchez-Rico M, Burgun A, Limosin F. Prevalence of contraindications to nirmatrelvir-ritonavir among hospitalized patients with COVID-19 at risk for progression to severe disease. JAMA Netw Open. 2022; 5(11):e2242140. PMID: 36378313.19. Lim S, Tignanelli CJ, Hoertel N, Boulware DR, Usher MG. Prevalence of medical contraindications to nirmatrelvir/ritonavir in a cohort of hospitalized and nonhospitalized patients with COVID-19. Open Forum Infect Dis. 2022; 9(8):ofac389. PMID: 36000003.20. Kim MK, Lee KS, Ham SY, Choi YY, Lee E, Lee S, et al. Real-world effectiveness of nirmatrelvir-ritonavir and its acceptability in high-risk COVID-19 patients. J Korean Med Sci. 2023; 38(35):e272. PMID: 37667578.21. Petrilli CM, Jones SA, Yang J, Rajagopalan H, O’Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020; 369:m1966. PMID: 32444366.22. Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020; 584(7821):430–436. PMID: 32640463.23. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020; 395(10229):1054–1062. PMID: 32171076.24. Skarbinski J, Wood MS, Chervo TC, Schapiro JM, Elkin EP, Valice E, et al. Risk of severe clinical outcomes among persons with SARS-CoV-2 infection with differing levels of vaccination during widespread Omicron (B.1.1.529) and Delta (B.1.617.2) variant circulation in Northern California: a retrospective cohort study. Lancet Reg Health Am. 2022; 12:100297. PMID: 35756977.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effectiveness and Adverse Events of Nirmatrelvir/Ritonavir Versus Molnupiravir for COVID-19 in Outpatient Setting: Multicenter Prospective Observational Study
- Nirmatrelvir/Ritonavir Prescription Rate and Outcomes in Coronavirus Disease 2019: A Single Center Study
- Treatment Options for Patients With Mild-to-Moderate Coronavirus Disease 2019 in Korea
- Safety Monitoring of Oral Antiviral COVID-19 Treatment Using Korea Adverse Event Reporting System (KAERS) Database
- Nationwide Target Trial Emulation Evaluating the Clinical Effectiveness of Oral Antivirals for COVID-19 in Korea