J Korean Med Sci.
2022 Dec;37(48):e352. 10.3346/jkms.2022.37.e352.
Treatment Options for Patients With Mild-to-Moderate Coronavirus Disease 2019 in Korea
- Affiliations
-
- 1Research Institute for Public Healthcare, National Medical Center, Seoul, Korea
- 2Department of Infectious Diseases, Clinical Infectious Disease Research Center, National Medical Center, Seoul, Korea
- 3Division of Infectious Diseases, Department of Internal Medicine, National Medical Center, Seoul, Korea
- KMID: 2536734
- DOI: http://doi.org/10.3346/jkms.2022.37.e352
Abstract
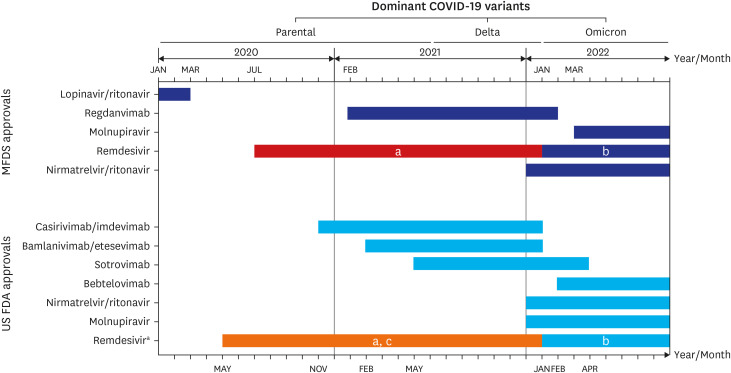
- The coronavirus disease 2019 (COVID-19) continues to threaten public health in Korea although several surges have passed in the past 3 years since 2019. Although patients with mild-to-moderate COVID-19 can usually recover at home, antiviral therapy to prevent disease progression and hospitalization is beneficial for those at high risk of progressing to severe COVID-19. The purpose of this article was to review how antivirals have been rolled out for the treatment of COVID-19 and how domestic and international guidelines for their use have evolved. Several evidence-based treatment guidelines have been developed in Korea, including those derived from domestic studies. Although many different antiviral agents were nominated as promising therapeutics at the onset of the pandemic, most failed to show efficacy in clinical trials. Currently, three types of antiviral agents—nirmatrelvir-ritonavir, molnupiravir, and remdesivir—are available in Korea to treat severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Each antiviral has its advantages and disadvantages. For most individuals, nirmatrelvir/ritonavir is preferred because of its high efficacy and convenience of administration. When serious drug interactions occur or are expected with nirmatrelvir/ritonavir administration, 3 days of remdesivir treatment is shown to be a reasonable alternative. Molnupiravir may be prescribed with caution only if no other therapeutic options are available or acceptable.
Keyword
Figure
Cited by 2 articles
-
Risk Factors for the Prescription of Ineffective Antiviral Candidates for COVID-19 During the Early Pandemic Period in Korea
Eunyoung Lee, Seungyeon Kim, Sun Young Lee, Joo Jeong, Jihwan Bang, Juhwan Oh, Sang Do Shin, Nam Joong Kim, Pyoeng Gyun Choe, Myoung-don Oh
J Korean Med Sci. 2023;38(36):e280. doi: 10.3346/jkms.2023.38.e280.Adverse Drug Events Associated With Remdesivir in Real-World Hospitalized Patients With COVID-19, Including Vulnerable Populations: A Retrospective Multicenter Study
Hyein Kang, Chang Kyung Kang, Jae Hyoung Im, Yoonsook Cho, Dong Yoon Kang, Ju-Yeun Lee
J Korean Med Sci. 2023;38(44):e346. doi: 10.3346/jkms.2023.38.e346.
Reference
-
1. World Health Organization (WHO). Coronavirus (COVID-19) dashboard. Updated 2022. Accessed October 26, 2022. https://covid19.who.int/ .2. Korea Disease Control and Prevention Agency. Daily and cumulative number of confirmed cases. Updated October 30, 2022. Accessed October 30, 2022. https://ncov.kdca.go.kr/en/bdBoardList.do?brdId=16&brdGubun=161&dataGubun=&ncvContSeq=&contSeq=&board_id=&gubun= .3. Korea Disease Control and Prevention Agency. The status of genomic surveillance of the COVID-19 variant virus. Updated October 20, 2022. Accessed October 29, 2022. https://kdca.go.kr/contents.es?mid=a20107030000 .4. Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid-19. N Engl J Med. 2020; 383(18):1757–1766. PMID: 32329974.5. Gandhi RT, Malani PN, Del Rio C. COVID-19 therapeutics for nonhospitalized patients. JAMA. 2022; 327(7):617–618. PMID: 35029659.6. Kim SB, Ryoo S, Huh K, Joo EJ, Kim YJ, Choi WS, et al. Revised Korean Society of Infectious Diseases/National Evidence-based Healthcarea Collaborating Agency guidelines on the treatment of patients with COVID-19. Infect Chemother. 2021; 53(1):166–219. PMID: 34409790.7. Kwak MY, Jo EY, Chin B, Park SE, Yim J, Lee JE, et al. Development and roll-out of a coronavirus disease 2019 clinical pathway for standardized qualified care in public hospitals in Korea. Infect Chemother. 2022; 54(2):353–359. PMID: 35794720.8. National Institutes of Health. Coronavirus disease 2019 (COVID-19) treatment guidelines. Updated October 19, 2022. Accessed October 29, 2022. https://www.covid19treatmentguidelines.nih.gov/ .9. Sanchez-Gonzalez MA, Moskowitz D, Issuree PD, Yatzkan G, Rizvi SA, Day K. A pathophysiological perspective on COVID-19’s lethal complication: from viremia to hypersensitivity pneumonitis-like immune dysregulation. Infect Chemother. 2020; 52(3):335–344. PMID: 32537960.10. Vegivinti CT, Evanson KW, Lyons H, Akosman I, Barrett A, Hardy N, et al. Efficacy of antiviral therapies for COVID-19: a systematic review of randomized controlled trials. BMC Infect Dis. 2022; 22(1):107. PMID: 35100985.11. Kim SB, Huh K, Heo JY, Joo EJ, Kim YJ, Choi WS, et al. Interim guidelines on antiviral therapy for COVID-19. Infect Chemother. 2020; 52(2):281–304. PMID: 32342676.12. Seo H, Kim H, Bae S, Park S, Chung H, Sung HS, et al. Fluvoxamine treatment of patients with symptomatic COVID-19 in a community treatment center: a preliminary result of randomized controlled trial. Infect Chemother. 2022; 54(1):102–113. PMID: 35384422.13. Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020; 383(19):1813–1826. PMID: 32445440.14. Korea Ministry of Food and Drug Safety. Special import approved for COVID-19 treatment remdesivir. Updated June 3, 2020. Accessed October 29, 2022. https://www.mfds.go.kr/eng/brd/m_64/view.do?seq=30&srchFr=&srchTo=&srchWord=remdesivir&srchTp=7&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=1 .15. WHO Solidarity Trial Consortium. Remdesivir and three other drugs for hospitalised patients with COVID-19: final results of the WHO Solidarity randomised trial and updated meta-analyses. Lancet. 2022; 399(10339):1941–1953. PMID: 35512728.16. Joo EJ, Ko JH, Kim SE, Kang SJ, Baek JH, Heo EY, et al. Clinical and virologic effectiveness of remdesivir treatment for severe coronavirus disease 2019 (COVID-19) in Korea: a nationwide multicenter retrospective cohort study. J Korean Med Sci. 2021; 36(11):e83. PMID: 33754512.17. Korea Ministry of Food and Drug Safety. Additional approval for emergency use of remdesivir. Updated January 20, 2022. Accessed October 29, 2022. https://www.mfds.go.kr/brd/m_99/view.do?seq=46086&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=1 .18. Gottlieb RL, Vaca CE, Paredes R, Mera J, Webb BJ, Perez G, et al. Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med. 2022; 386(4):305–315. PMID: 34937145.19. Ader F, Bouscambert-Duchamp M, Hites M, Peiffer-Smadja N, Poissy J, Belhadi D, et al. Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial. Lancet Infect Dis. 2022; 22(2):209–221. PMID: 34534511.20. Owen DR, Allerton CM, Anderson AS, Aschenbrenner L, Avery M, Berritt S, et al. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science. 2021; 374(6575):1586–1593. PMID: 34726479.21. Hammond J, Leister-Tebbe H, Gardner A, Abreu P, Bao W, Wisemandle W, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022; 386(15):1397–1408. PMID: 35172054.22. The United States Food and Drug Administration. Fact sheet for healthcare providers: emergency use authorization for paxlovid™. Updated September 2022. Accessed October 29, 2022. https://www.fda.gov/media/155050/download .23. Korea Ministry of Food and Drug Safety. Emergency use authorization of paxlovid™. Updated December 27, 2021. Accessed October 29, 2022. https://www.mfds.go.kr/brd/m_99/view.do?seq=46032&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=1 .24. Park H, Lee HY, Yu M, Song YJ, Lee SE, Park YJ, et al. Effectiveness of COVID-19 vaccine and Paxlovid treatment against SARS-Cov-2 infection related severe outcome and death during the Omicron variant outbreak; COV-EPI evaluation study in LTCFs. Public Health Wkly Rep. 2022; 15(24):1688–1695.25. Painter WP, Holman W, Bush JA, Almazedi F, Malik H, Eraut NC, et al. Human safety, tolerability, and pharmacokinetics of molnupiravir, a novel broad-spectrum oral antiviral agent with activity against SARS-CoV-2. Antimicrob Agents Chemother. 2021; 65(5):e02428-20. PMID: 33649113.26. Yoo JH. Antivirals for coexistence with COVID-19: brief review for general physicians. J Korean Med Sci. 2021; 36(42):e298. PMID: 34725982.27. Fischer WA 2nd, Eron JJ Jr, Holman W, Cohen MS, Fang L, Szewczyk LJ, et al. A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus. Sci Transl Med. 2022; 14(628):eabl7430. PMID: 34941423.28. Jayk Bernal A, Gomes da Silva MM, Musungaie DB, Kovalchuk E, Gonzalez A, Delos Reyes V, et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med. 2022; 386(6):509–520. PMID: 34914868.29. Ministry of Food and Drug Safety (MFDS). Emergency use authorization of lagevrio™. Updated March 23, 2022. Accessed October 29, 2022. https://impfood.mfds.go.kr/CFBBB02F02/getCntntsDetail?cntntsSn=462249 .30. Jeong JH, Chokkakula S, Min SC, Kim BK, Choi WS, Oh S, et al. Combination therapy with nirmatrelvir and molnupiravir improves the survival of SARS-CoV-2 infected mice. Antiviral Res. 2022; 208:105430. PMID: 36209984.31. Jo Y, Kim SB, Radnaabaatar M, Huh K, Yoo JH, Peck KR, et al. Model-based cost-effectiveness analysis of oral antivirals against SARS-CoV-2 in Korea. Epidemiol Health. 2022; 44:e2022034. PMID: 35381167.32. Zhou S, Hill CS, Sarkar S, Tse LV, Woodburn BM, Schinazi RF, et al. β-d-N4-hydroxycytidine inhibits SARS-CoV-2 through lethal mutagenesis but is also mutagenic to mammalian cells. J Infect Dis. 2021; 224(3):415–419. PMID: 33961695.33. Dyer O. Covid-19: FDA expert panel recommends authorising molnupiravir but also voices concerns. BMJ. 2021; 375(2984):n2984. PMID: 34857644.34. Hong SH, Shi HJ, Kim SY, Park Y, Eom JS. Clinical characteristics and pregnancy-related outcomes of pregnant women hospitalized with COVID-19 during the delta wave: a single-center observational study. Infect Chemother. 2022; 54(3):433–445. PMID: 35920268.35. National Evidence-based Healthcare Collaborating Agency. COVID-19 living guidelines. Updated August 24, 2022. Accessed October 29, 2022. https://www.neca.re.kr/lay1/bbs/S1T11C174/F/58/view.do?article_seq=8932&cpage=1&rows=10&condition=&keyword=&show=&cat= .36. VanBlargan LA, Errico JM, Halfmann PJ, Zost SJ, Crowe JE Jr, Purcell LA, et al. An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies. Nat Med. 2022; 28(3):490–495. PMID: 35046573.37. Syed YY. Regdanvimab: first approval. Drugs. 2021; 81(18):2133–2137. PMID: 34724174.38. Choi SJ, Park SW, Lee E. Effectiveness of regdanvimab at preventing the need for oxygen therapy in patients with mild-to-moderate COVID-19: a retrospective cohort study. Infect Chemother. 2022; 54(1):91–101. PMID: 35384421.39. Hong SI, Ryu BH, Hong KW, Bae IG, Cho OH. Real world experience with regdanvimab treatment of mild-to-moderate coronavirus disease-19 in a COVID-19 designated hospital of Korea. Infect Chemother. 2022; 54(1):114–124. PMID: 35384423.40. Kwak YG, Song JE, Kang J, Kang J, Kang HK, Koo HK, et al. Use of the monoclonal antibody regdanvimab to treat patients hospitalized with COVID-19: real-world data during the delta variant predominance. Infect Chemother. 2022; 54:e66.41. Ryu DK, Kang B, Noh H, Woo SJ, Lee MH, Nuijten PM, et al. The in vitro and in vivo efficacy of CT-P59 against Gamma, Delta and its associated variants of SARS-CoV-2. Biochem Biophys Res Commun. 2021; 578:91–96. PMID: 34547629.42. Korea Disease Control and Prevention Agency. Korea Disease Control and Prevention Agency COVID-19 regular briefing. Updated February 24, 2022. Accessed 29 October, 2022. https://www.korea.kr/news/policyBriefingView.do?newsId=156497146 .
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical and Epidemiological Characteristics of Coronavirus Disease 2019 in the Early Stage of Outbreak
- Therapeutics for the treatment of coronavirus disease 2019 in children and adolescents
- Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)-Associated Urogenital Disease: A Current Update
- Treatment Options for Severe Acute Respiratory Syndrome, Middle East Respiratory Syndrome, and Coronavirus Disease 2019: a Review of Clinical Evidence
- Real World Experience with Regdanvimab Treatment of Mild-toModerate Coronavirus Disease-19 in a COVID-19 Designated Hospital of Korea