J Korean Neurosurg Soc.
2024 Mar;67(2):158-165. 10.3340/jkns.2023.0125.
Assessing the Adequacy of Superficial Temporal Artery Blood Flow in Korean Patients Undergoing STA-MCA Anastomosis
- Affiliations
-
- 1Department of Neurosurgery, Eunpyeong St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Department of Neurosurgery, Bucheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2553121
- DOI: http://doi.org/10.3340/jkns.2023.0125
Abstract
Objective
: Superficial temporal artery (STA)-middle cerebral artery (MCA) anastomosis is conducted for flow augmentation. In this study, we measured the STA cut flow of a Korean population and evaluated the relationship between STA cut flow and long-term patency of the bypass.
Methods
: A retrospective study was conducted. Intraoperative measurement of STA flow was conducted using a microvascular flow meter on patients who underwent STA-MCA. After cutting the distal end, the STA flow rate was measured with no resistance and recorded. After finishing anastomosis, STA flow was measured and recorded. The cut flow index was calculated by dividing post anastomosis flow by cut flow in intracranial atherosclerotic stenosis patients.
Results
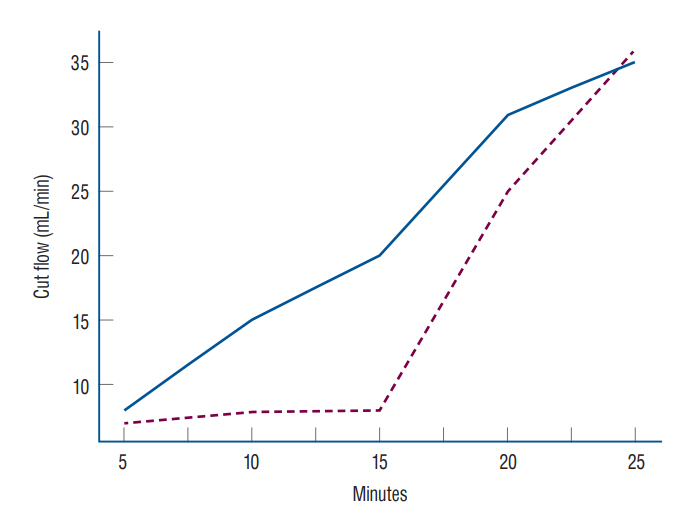
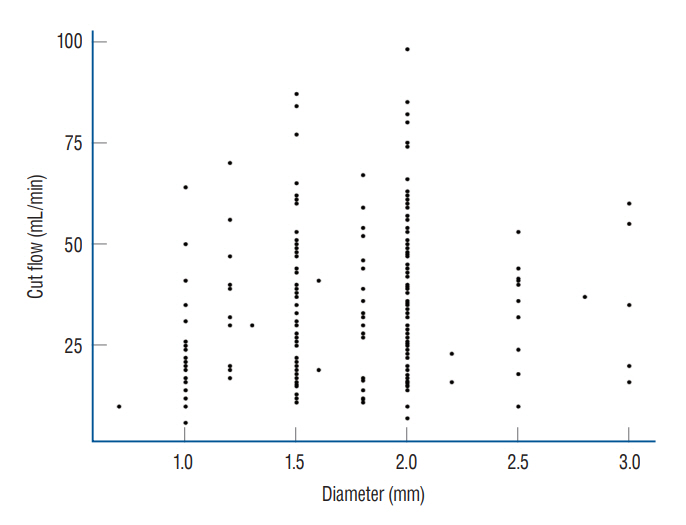
: The median STA cut flow was 35.0 mL/min and the post anastomosis flow was 24.0 mL/min. The cut flow of STA decreased with aging (p=0.027) and increased with diameter (p=0.004). The cut flow showed no correlation with history of hypertension or diabetes mellitus (p=0.713 and p=0.786), but did correlate a positively with history of hyperlipidemia (p=0.004). There were no statistical differences in cut flow, STA diameter, and post anastomosis flow between the frontal and parietal branches (p=0.081, p=0.853, and p=0.990, respectively).
Conclusion
: The median STA cut flow of a Korean population was 35 mL/min. Upon reviewing previous articles, it appears that there are differences in the STA cut flow between Western and Asian patients.
Figure
Reference
-
References
1. Amin-Hanjani S, Du X, Mlinarevich N, Meglio G, Zhao M, Charbel FT. The cut flow index: an intraoperative predictor of the success of extracranial-intracranial bypass for occlusive cerebrovascular disease. Neurosurgery. 56(1 Suppl):75–85. discussion 75-85. 2005.2. Beverly JK, Budoff MJ. Atherosclerosis: pathophysiology of insulin resistance, hyperglycemia, hyperlipidemia, and inflammation. J Diabetes. 12:102–104. 2020.3. Hage ZA, Behbahani M, Amin-Hanjani S, Charbel FT. Carotid bypass for carotid occlusion. Curr Atheroscler Rep. 17:36. 2015.4. Helthuis JHG, van Doormaal TPC, Amin-Hanjani S, Du X, Charbel FT, Hillen B, et al. A patient-specific cerebral blood flow model. J Biomech. 98:109445. 2020.5. Kuriyama S, Kusaka Y, Fujimura M, Wakai K, Tamakoshi A, Hashimoto S, et al. Prevalence and clinicoepidemiological features of moyamoya disease in Japan: findings from a nationwide epidemiological survey. Stroke. 39:42–47. 2008.6. Liu JK, Kan P, Karwande SV, Couldwell WT. Conduits for cerebrovascular bypass and lessons learned from the cardiovascular experience. Neurosurg Focus. 14:e3. 2003.7. Markert MS, Della-Morte D, Cabral D, Roberts EL Jr, Gardener H, Dong C, et al. Ethnic differences in carotid artery diameter and stiffness: the Northern Manhattan study. Atherosclerosis. 219:827–832. 2011.8. Matsukawa H, Tanikawa R, Kamiyama H, Tsuboi T, Noda K, Ota N, et al. The valveless saphenous vein graft technique for EC-IC high-flow bypass: technical note. World Neurosurg. 87:35–38. 2016.9. Mohit AA, Sekhar LN, Natarajan SK, Britz GW, Ghodke B. High-flow bypass grafts in the management of complex intracranial aneurysm. Neurosurgery. 60(2 Suppl 1):ONS105–ONS122. discussion ONS122-ONS123. 2007.10. Okada Y, Shima T, Nishida M, Yamane K, Yamada T, Yamanaka C. Effectiveness of superficial temporal artery-middle cerebral artery anastomosis in adult moyamoya disease: cerebral hemodynamics and clinical course in ischemic and hemorrhagic varieties. Stroke. 29:625–630. 1998.11. Sekhar LN, Natarajan SK, Ellenbogen RG, Ghodke B. Cerebral revascularization for ischemia, aneurysms, and cranial base tumors. Neurosurgery. 62(6 Suppl 3):SHC1373–SHC1410. discussion 1408-1410. 2008.12. Stapleton CJ, Atwal GS, Hussein AE, Amin-Hanjani S, Charbel FT. The cut flow index revisited: utility of intraoperative blood flow measurements in extracranial-intracranial bypass surgery for ischemic cerebrovascular disease. J Neurosurg. 133:1396–1400. 2019.13. Tulleken CA, van der Zwan A, Kappelle LJ. High-flow transcranial bypass for prevention of brain ischemia. Ned Tijdschr Geneeskd. 143:2281–2285. 1999.14. Unda SR, Antoniazzi AM, Fluss R, Yassari N, Esenwa C, Haranhalli N, et al. Ethnic-associated phenotype variations in moyamoya cerebrovascular outcomes. Cerebrovasc Dis. 52:519–525. 2023.15. Wang G, Zhang X, Wang B, Wen Y, Chen S, Liu J, et al. Flow evaluation of STA-MCA bypass using quantitative ultrasonography: an alternative to standard angiography for follow up of bypass graft. J Stroke Cerebrovasc Dis. 29:105000. 2020.16. Zechariah A, ElAli A, Hagemann N, Jin F, Doeppner TR, Helfrich I, et al. Hyperlipidemia attenuates vascular endothelial growth factor-induced angiogenesis, impairs cerebral blood flow, and disturbs stroke recovery via decreased pericyte coverage of brain endothelial cells. Arterioscler Thromb Vasc Biol. 33:1561–1567. 2013.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Changes in Bypass Flow during Temporary Occlusion of Unused Branch of Superficial Temporal Artery
- Superficial Temporal Artery-Middle Cerebral Artery Anastomosis for Internal Carotid Artery Occlusion by Subacute In-Stent Thrombosis after Carotid Artery Stenting
- Emergency In Situ Bypass during Middle Cerebral Artery Aneurysm Surgery: Middle Cerebral Artery-Superficial Temporal Artery Interposition Graft-Middle Cerebral Artery Anastomosis
- Extracranial-Intracranial Bypass Surgery: Surgical Techniques and Perioperative Management
- A Middle Cerebral Artery AneurysmOriginating Near the Site of Anastomosis after Superficial Temporal Artery-Middle Cerebral Artery Bypass: Case Report