Ann Pediatr Endocrinol Metab.
2024 Feb;29(1):46-53. 10.6065/apem.2346028.014.
Effectiveness and safety of pamidronate treatment in nonambulatory children with low bone mineral density
- Affiliations
-
- 1Department of Pediatrics, Severance Children’s Hospital, Endocrine Research Institute, Yonsei University College of Medicine, Seoul, Korea
- 2Department of Pediatrics, CHA Gangnam Medical Center, CHA University, Seoul, Korea
- 3Department of Pediatrics, International St. Mary’s Hospital, Catholic Kwandong University, Incheon, Korea
- KMID: 2553017
- DOI: http://doi.org/10.6065/apem.2346028.014
Abstract
- Purpose
Nonambulatory pediatric patients may have low bone mineral density (BMD) and increased risk of pathologic fractures. Though bisphosphonate therapy is the mainstream medical intervention in these children, clinical data regarding this treatment are limited. Therefore, this study aimed to evaluate the effectiveness and safety of bisphosphonate therapy in such children.
Methods
We conducted a retrospective study of 21 nonambulatory children (Gross Motor Function Classification System level V) with BMD z-score ≤ -2.0 who were treated with intravenous pamidronate for at least 1 year. These patients received pamidronate every 4 months at a dose of 1.0 to 3.0 mg/kg for each cycle and had regular follow-ups for at least 1 year. The main outcome measures were changes in BMD, risk rate of fracture, biochemical data, and adverse events.
Results
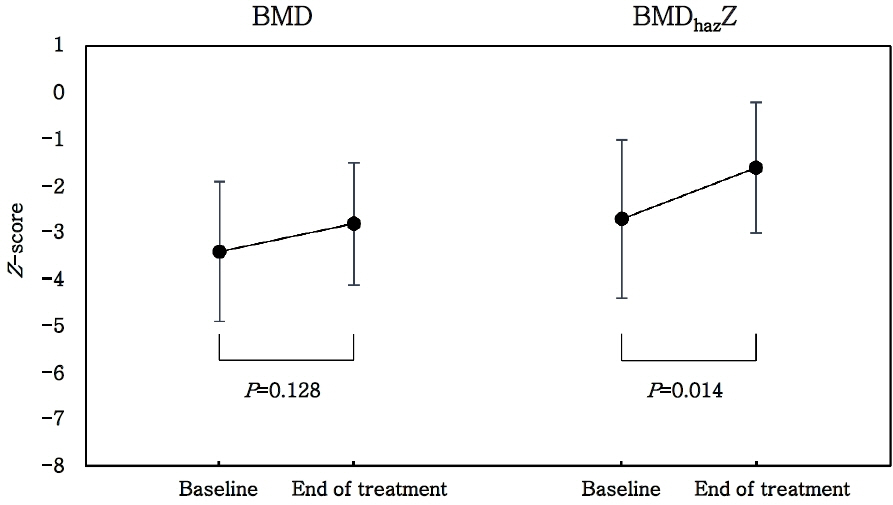
The average duration of pamidronate treatment was 2.0±0.9 years, and the mean cumulative dose of pamidronate according to body weight was 7.7±2.5 mg/kg/yr. After treatment, the mean lumbar spine bone mineral content, BMD, and height-for-age-z-score-adjusted BMD z-score (BMDhazZ) significantly improved. The relative risk of fracture after treatment was 0.21 (p=0.0032), suggesting that pamidronate treatment reduced fracture incidence significantly. The increase in the average dose per body weight in each cycle significantly increased the changes in BMDhazZ.
Conclusion
Pamidronate treatment improved the bone health of nonambulatory children with low bone density without any significant adverse events. Independent of cumulative dosage and duration of treatment, the effectiveness of pamidronate increased significantly with an increase in the average dose per body weight in subsequent cycles.
Keyword
Figure
Reference
-
References
1. Bishop N, Arundel P, Clark E, Dimitri P, Farr J, Jones G, et al. Fracture prediction and the definition of osteoporosis in children and adolescents: the ISCD 2013 Pediatric Official Positions. J Clin Densitom. 2014; 17:275–80.
Article2. Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet. 2019; 393:364–76.
Article3. Henderson RC. Bone density and other possible predictors of fracture risk in children and adolescents with spastic quadriplegia. Dev Med Child Neurol. 1997; 39:224–7.
Article4. Lee JJ, Lyne ED. Pathologic fractures in severely handicapped children and young adults. J Pediatr Orthop. 1990; 10:497–500.
Article5. Song K, Kwon A, Chae HW, Suh J, Choi HS, Choi Y, et al. Vitamin D status is associated with bone mineral density in adolescents: findings from the Korea National Health and Nutrition Examination Survey. Nutr Res. 2021; 87:13–21.
Article6. Henderson RC, Lark RK, Gurka MJ, Worley G, Fung EB, Conaway M, et al. Bone density and metabolism in children and adolescents with moderate to severe cerebral palsy. Pediatrics. 2002; 110:e5.
Article7. Berger C, Goltzman D, Langsetmo L, Joseph L, Jackson S, Kreiger N, et al. Peak bone mass from longitudinal data: implications for the prevalence, pathophysiology, and diagnosis of osteoporosis. J Bone Miner Res. 2010; 25:1948–57.
Article8. Golden NH, Abrams SA, Committee on N. Optimizing bone health in children and adolescents. Pediatrics. 2014; 134:e1229–43.
Article9. Weaver CM, Gordon CM, Janz KF, Kalkwarf HJ, Lappe JM, Lewis R, et al. The National Osteoporosis Foundation's position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporos Int. 2016; 27:1281–386.
Article10. Bachrach LK, Ward LM. Clinical review 1: bisphosphonate use in childhood osteoporosis. J Clin Endocrinol Metab. 2009; 94:400–9.11. Camacho PM, Petak SM, Binkley N, Diab DL, Eldeiry LS, Farooki A, et al. American Association of Clinical Endocrinologists/American College of Endocrinology Clinical Practice Guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2020 update. Endocr Pract. 2020; 26:1–46.
Article12. Glorieux FH, Bishop NJ, Plotkin H, Chabot G, Lanoue G, Travers R. Cyclic administration of pamidronate in children with severe osteogenesis imperfecta. N Engl J Med. 1998; 339:947–52.
Article13. Henderson RC, Lark RK, Kecskemethy HH, Miller F, Harcke HT, Bachrach SJ. Bisphosphonates to treat osteopenia in children with quadriplegic cerebral palsy: a randomized, placebo-controlled clinical trial. J Pediatr. 2002; 141:644–51.
Article14. Grissom LE, Kecskemethy HH, Bachrach SJ, McKay C, Harcke HT. Bone densitometry in pediatric patients treated with pamidronate. Pediatr Radiol. 2005; 35:511–7.
Article15. Moon SJ, An YM, Kim SK, Kwon YS, Lee JE. The effect of low-dose intravenous bisphosphonate treatment on osteoporosis in children with quadriplegic cerebral palsy. Korean J Pediatr. 2017; 60:403–7.
Article16. Yoon JH, Choi Y, Lee Y, Yoo HW, Choi JH. Efficacy and safety of intravenous pamidronate infusion for treating osteoporosis in children and adolescents. Ann Pediatr Endocrinol Metab. 2021; 26:105–11.
Article17. Bachrach SJ, Kecskemethy HH, Harcke HT, Hossain J. Decreased fracture incidence after 1 year of pamidronate treatment in children with spastic quadriplegic cerebral palsy. Dev Med Child Neurol. 2010; 52:837–42.
Article18. Simm PJ, Biggin A, Zacharin MR, Rodda CP, Tham E, Siafarikas A, et al. Consensus guidelines on the use of bisphosphonate therapy in children and adolescents. J Paediatr Child Health. 2018; 54:223–33.
Article19. Kim JH, Yun S, Hwang SS, Shim JO, Chae HW, Lee YJ, et al. The 2017 Korean National Growth Charts for children and adolescents: development, improvement, and prospects. Korean J Pediatr. 2018; 61:135–49.
Article20. Munns CF, Shaw N, Kiely M, Specker BL, Thacher TD, Ozono K, et al. Global consensus recommendations on prevention and management of nutritional rickets. J Clin Endocrinol Metab. 2016; 101:394–415.21. Zemel BS, Leonard MB, Kelly A, Lappe JM, Gilsanz V, Oberfield S, et al. Height adjustment in assessing dual energy x-ray absorptiometry measurements of bone mass and density in children. J Clin Endocrinol Metab. 2010; 95:1265–73.
Article22. Rolvien T, Amling M. Disuse Osteoporosis: clinical and mechanistic insights. Calcif Tissue Int. 2022; 110:592–604.
Article23. Leblanc AD, Schneider VS, Evans HJ, Engelbretson DA, Krebs JM. Bone mineral loss and recovery after 17 weeks of bed rest. J Bone Miner Res. 1990; 5:843–50.
Article24. Qin L, Liu W, Cao H, Xiao G. Molecular mechanosensors in osteocytes. Bone Res. 2020; 8:23.
Article25. Pack AM. Bone disease in epilepsy. Curr Neurol Neurosci Rep. 2004; 4:329–34.26. Henderson RC. Vitamin D levels in noninstitutionalized children with cerebral palsy. J Child Neurol. 1997; 12:443–7.
Article27. Plotkin H, Coughlin S, Kreikemeier R, Heldt K, Bruzoni M, Lerner G. Low doses of pamidronate to treat osteopenia in children with severe cerebral palsy: a pilot study. Dev Med Child Neurol. 2006; 48:709–12.
Article28. Mallmin H, Ljunghall S, Larsson K, Lindh E. Short-term effects of pamidronate on biochemical markers of bone metabolism in osteoporosis--a placebo-controlled dosefinding study. Ups J Med Sci. 1991; 96:205–12.
Article29. Li P, Zhao Z, Wang L, Jin X, Shen Y, Nan C, et al. Minimally effective concentration of zoledronic acid to suppress osteoclasts in vitro. Exp Ther Med. 2018; 15:5330–6.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy of pamidronate in children with low bone mineral density during and after chemotherapy for acute lymphoblastic leukemia and non-Hodgkin lymphoma
- Efficacy and safety of intravenous pamidronate infusion for treating osteoporosis in children and adolescents
- Efficacy of pamidronate in pediatric osteosarcoma patients with low bone mineral density
- Effect of cyclic pamidronate administration on osteoporosis in children with β-thalassemia major: a single-center study
- Short-term Efficacy of Monthly Pamidronate Infusion in Patients with Osteogenesis Imperfecta