Anesth Pain Med.
2024 Jan;19(1):62-67. 10.17085/apm.23124.
Preoperative evaluation of systolic murmur with point-of-care echocardiography before an elective thoracic surgery - A case report -
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Konkuk University Medical Center, Seoul, Korea
- 2Department of Anesthesiology and Pain Medicine, Konkuk University School of Medicine, Seoul, Korea
- KMID: 2552858
- DOI: http://doi.org/10.17085/apm.23124
Abstract
- Background
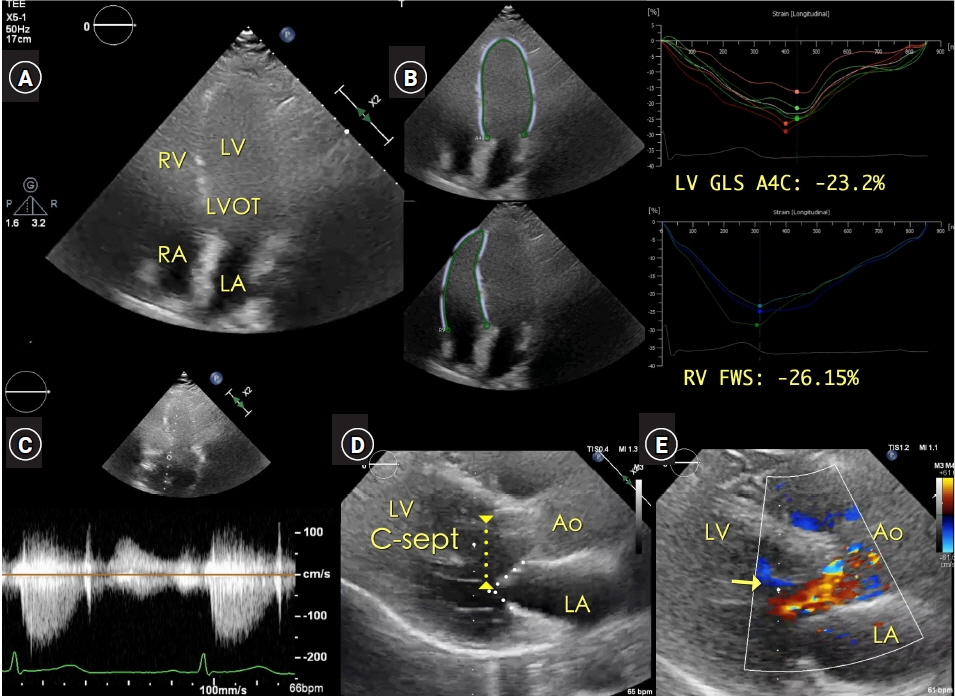
Systolic murmur suggesting the association of aortic valve (AV) stenosis or obstructive pathology in the left ventricular outflow tract (LVOT) usually requires preoperative echocardiographic evaluation for elective surgery. Case: In a 63-year-old female patient undergoing elective thoracic surgery, the systolic murmur was auscultated on the right sternal border of the second intercostal space in the preoperative patient holding area. Point-of-care (POC) transthoracic echocardiography (TTE) demonstrated a systolic jet flow in the LVOT area. The peak systolic velocity of the continuous wave Doppler tracing, aligned to the LVOT and the AV, was approximately 1.5 m/s. The peak/mean pressure gradient was 11/6 mmHg for the AV and 9/5 mmHg for the LVOT. Anesthesia was induced under continuous TTE imaging. Intraoperative transesophageal echocardiography also confirmed the absence of any cardiac pathology.
Conclusions
POC echocardiography offered a thorough preoperative evaluation of an unexpectedly identified systolic murmur, avoiding a potential delay in the operation schedule for conventional preoperative echocardiographic evaluation.
Figure
Reference
-
1. Maslow AD, Regan MM, Haering JM, Johnson RG, Levine RA. Echocardiographic predictors of left ventricular outflow tract obstruction and systolic anterior motion of the mitral valve after mitral valve reconstruction for myxomatous valve disease. J Am Coll Cardiol. 1999; 34:2096–104.
Article2. Via G, Hussain A, Wells M, Reardon R, ElBarbary M, Noble VE, et al. International evidence-based recommendations for focused cardiac ultrasound. J Am Soc Echocardiogr. 2014; 27:683.e1–e33.
Article3. Kalagara H, Coker B, Gerstein NS, Kukreja P, Deriy L, Pierce A, et al. Point-of-Care Ultrasound (POCUS) for the cardiothoracic anesthesiologist. J Cardiothorac Vasc Anesth. 2022; 36:1132–47.
Article4. Ramsingh D, Bronshteyn YS, Haskins S, Zimmerman J. Perioperative Point-of-Care Ultrasound: from concept to application. Anesthesiology. 2020; 132:908–16.5. Roelandt JRTC. The decline of our physical examination skills: is echocardiography to blame? Eur Heart J Cardiovasc Imaging. 2014; 15:249–52.
Article6. Loxdale SJ, Sneyd JR, Donovan A, Werrett G, Viira DJ. The role of routine pre-operative bedside echocardiography in detecting aortic stenosis in patients with a hip fracture. Anaesthesia. 2012; 67:51–4.
Article7. Jiang L, Levine RA, King ME, Weyman AE. An integrated mechanism for systolic anterior motion of the mitral valve in hypertrophic cardiomyopathy based on echocardiographic observations. Am Heart J. 1987; 113:633–44.
Article8. Levine RA, Vlahakes GJ, Lefebvre X, Guerrero JL, Cape EG, Yoganathan AP, et al. Papillary muscle displacement causes systolic anterior motion of the mitral valve. Experimental validation and insights into the mechanism of subaortic obstruction. Circulation. 1995; 91:1189–95.
Article9. Klues HG, Roberts WC, Maron BJ. Morphological determinants of echocardiographic patterns of mitral valve systolic anterior motion in obstructive hypertrophic cardiomyopathy. Circulation. 1993; 87:1570–9.
Article10. Arbelo E, Protonotarios A, Gimeno JR, Arbustini E, Barriales-Villa R, Basso C, et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur Heart J. 2023; 44:3503–626.11. Houston BA, Stevens GR. Hypertrophic cardiomyopathy: a review. Clin Med Insights Cardiol. 2015; 8(Suppl 1):53–65.
Article12. Barber RL, Fletcher SN. A review of echocardiography in anaesthetic and peri-operative practice. Part 1: impact and utility. Anaesthesia. 2014; 69:764–76.
Article13. Halvorsen S, Mehilli J, Cassese S, Hall TS, Abdelhamid M, Barbato E, et al. 2022 ESC Guidelines on cardiovascular assessment and management of patients undergoing non-cardiac surgery. Eur Heart J. 2022; 43:3826–924.14. Canty DJ, Heiberg J, Yang Y, Royse AG, Margale S, Nanjappa N, et al. One-year results of the pilot multicentre randomised trial of preoperative focused cardiac ultrasound in hip fracture surgery. Anaesth Intensive Care. 2019; 47:207–8.
Article15. Chang JS, Ravi B, Jenkinson RJ, Paterson JM, Huang A, Pincus D. Impact of preoperative echocardiography on surgical delays and outcomes among adults with hip fracture. Bone Joint J. 2021; 103-B:271–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Musical murmur in a dog with acute chordae tendineae rupture
- Evaluation and diagnostic approach for heart murmurs in children
- Hypertrophic obstructive cardiomyopathy in a Yorkshire Terrier
- Preoperative cardiac evaluation with transthoracic echocardiography before non-cardiac surgery
- A case of situs inversus(I.D.D) with corrected TGA