Lab Med Online.
2023 Apr;13(2):65-71. 10.47429/lmo.2023.13.2.65.
Current Status and Perspectives for in vitro Medical Devices for Infectious Disease Diagnosis - Survey of Product Development and Clinical Trials
- Affiliations
-
- 1Department of Laboratory Medicine, Gyeongsang National University Changwon Hospital, Changwon, Korea
- 2Department of Laboratory Medicine , Gyeongsang National University, Jinju, Korea
- 3Gyeongsang Institute of Medical Science, Gyeongsang National University, Jinju, Korea
- KMID: 2552727
- DOI: http://doi.org/10.47429/lmo.2023.13.2.65
Abstract
- Background
The in vitro diagnostic (IVD) medical device industry has experienced remarkable growth during the COVID-19 pandemic. We here survey the existing difficulties in addressing various processes, including IVD development, clinical trials, domestic and overseas licensing, and areas in which improvement efforts and support should be focused.
Methods
A survey of companies registered in the Korea in vitro Diagnostic Association between November and December 2021was conducted using a Google questionnaire. In addition to basic questions about IVD development for infectious diseases and clinical trials, participants were asked for feedback regarding targeted infectious agents and hurdles faced in clinical trials. Twenty-three (52.3%) of 44 companies responded to our survey. For scoring, we either used the Likert scale or calculated the percentage of multiple answers.
Results
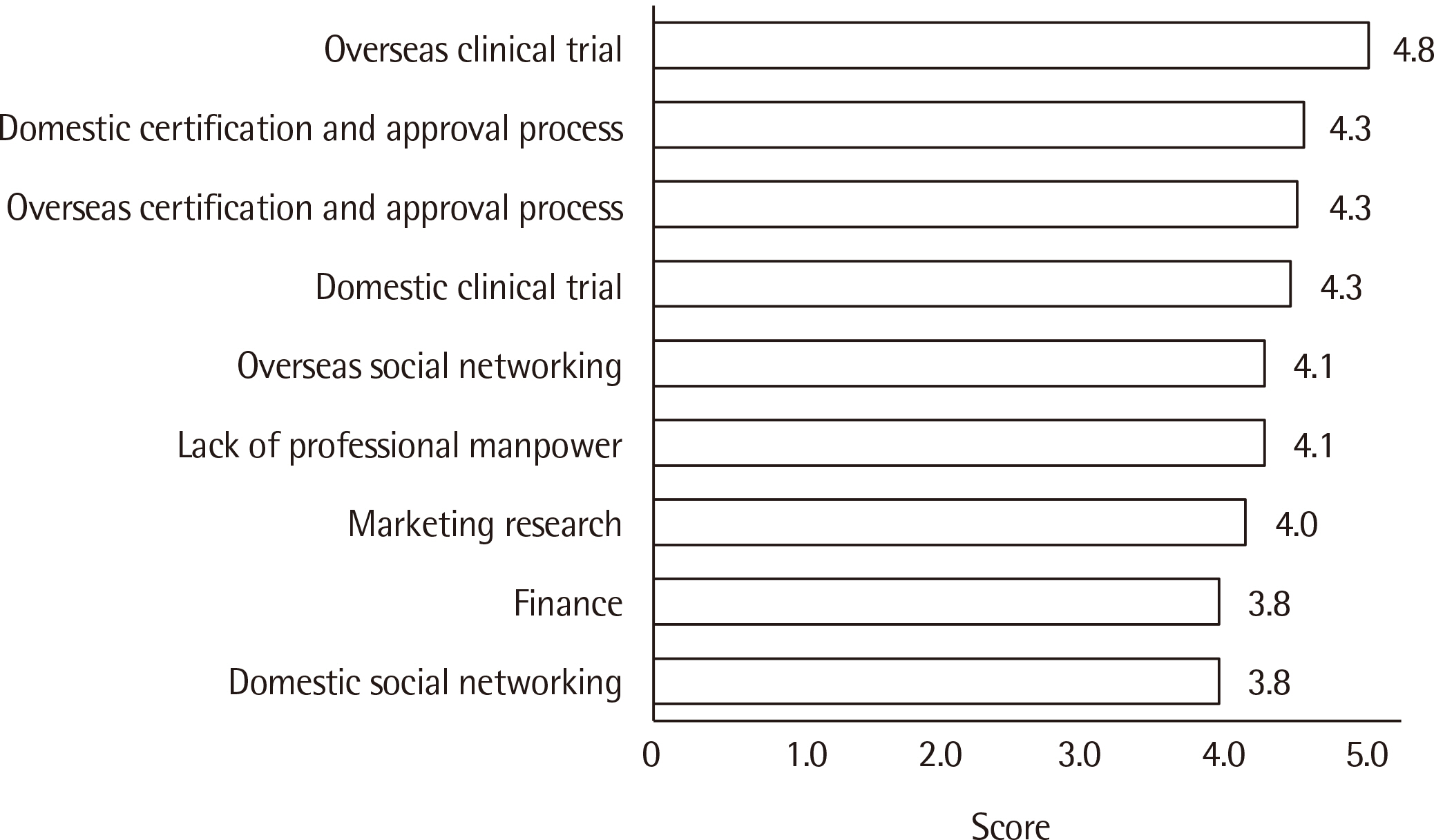
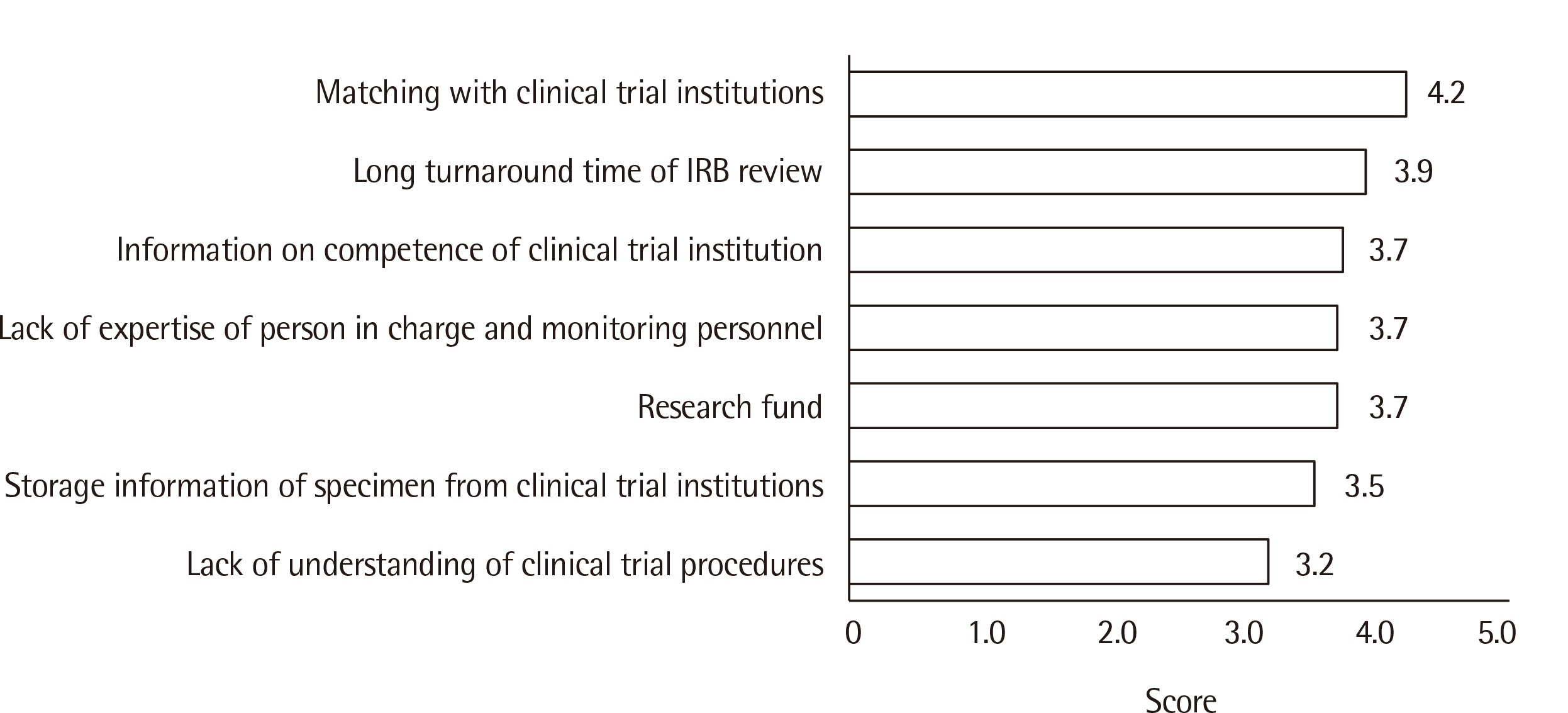
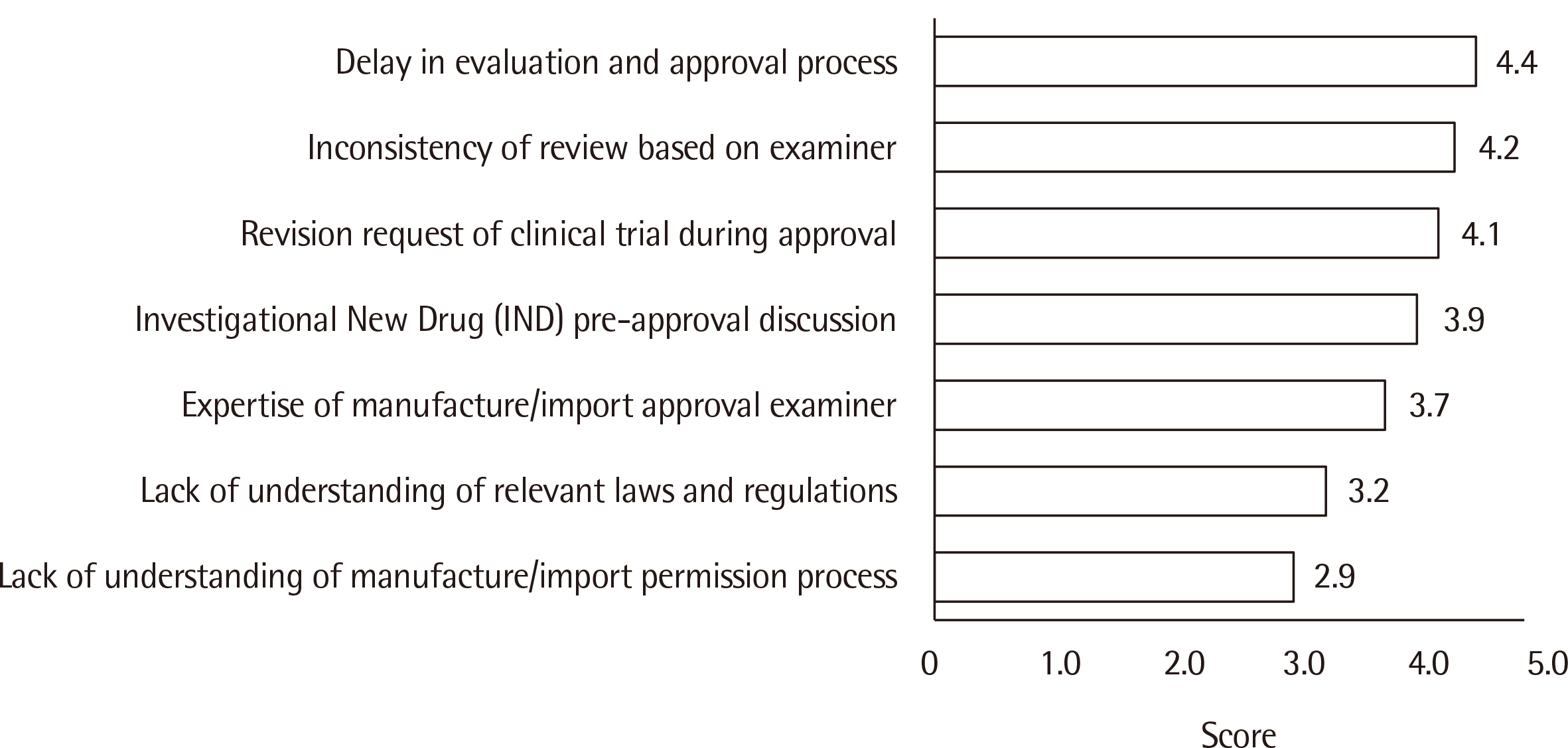
Matching with a clinical trial agency was the most difficult aspect of clinical trials, and delays in review and approval by the Korean Ministry of Food and Drug Safety (KMFDS) was a major challenge facing domestic licensing. Currently, the most actively targeted IVD development arena is COVID-19 (42.7%) followed by influenza (23.5%). The major purpose of clinical trials is to obtain KMFDS approval (91.3%), followed by European IVDR (in vitro Diagnostic Regulation) approval (60.9%).
Conclusions
The survey revealed that matching with well-prepared clinical trial institutions with sufficient stored clinical samples, accurate clinical performance analysis, and a rapid KMFDS review and approval process are necessary to enhance competitiveness and export.
Keyword
Figure
Cited by 1 articles
-
Experience of Approval of
In Vitro Diagnostic Medical Devices in Korea and Europe and Clinical Performance Study
Hwan Tae Lee, Jeong-Yeal Ahn, Ja Young Seo, Yiel-Hea Seo, Pil-Whan Park, Kyung-Hee Kim, Jaehyung You, Wookwan Park, Woo Jin Kim, Young Ho Yoon, Gwiyoung Oh, Daewon Kim
Lab Med Online. 2024;14(2):118-126. doi: 10.47429/lmo.2024.14.2.118.
Reference
-
1. Ministry of Food, Drug Safety. 2021. Food & Drug statistical yearbook 2021. GPRN 11-1471000-000165-10. https://www.mfds.go.kr/brd/m_371/down.do?brd_id=stat0016&seq=30720&data_tp=A&file_seq=1. Updated on Dec 2021. Choengju: Ministry of Food and Drug Safety.2. U.S. Department of Health and Human Services Food and Drug Administrations. Coronavirus Disease 2019 (COVID-19) Emergency Use Authorizations for Medical Devices. https://www.fda.gov/medical-devices/emergency-use-authorizations-medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices. Updated on Nov 2021.3. Ministry of Food, Drug Safety. First trade surplus for medical devices, continuous reinforcement of innovative regulatory services. https://www.mfds.go.kr/brd/m_99/view.do?seq=45489&srchFr=&srchTo=&srchWord=&srchTp=8&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&Data_stts_gubun=C9999&. Updated on Jun 2021.4. Korea Health Industry Development Institute. Industry analysis and policy research of medical device for diagnosis. KHIDI-CHIP-R-2020-1. https://www.khidi.or.kr/fileDownload?titleId=451530&fileId=1&fileDownType=C¶mMenuId=MENU02686. Updated on Jun 2021. Cheongju: Korea Health Industry Development Institute.5. Korea Health Industry Development Institute. Medical device industry analysis report 2020. https://www.khidi.or.kr/fileDownload?titleId=455915&fileId=1&fileDownType=C¶mMenuId=MENU00085. Updated on Oct 2021.6. Ministry of Food, Drug Safety. 2020 Medical device approval report. GPRN 11-1471000-000216-10. https://www.mfds.go.kr/brd/m_218/view.do?seq=33407&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=6. Updated on Aug 2021.7. Ravi N, Cortade DL, Ng E, Wang SX. 2020; Diagnostics for SARS-CoV-2 detection: A comprehensive review of the FDA-EUA COVID-19 testing landscape. Biosens Bioelectron. 165:112454. DOI: 10.1016/j.bios.2020.112454. PMID: 32729549. PMCID: PMC7368663.8. Dinnes J, Deeks JJ, Berhane S, Taylor M, Adriano A, Davenport C, et al. 2021; Rapid, point‐of‐care antigen and molecular‐based tests for diagnosis of SARS‐CoV‐2 infection. Cochrane Database of Syst Rev. 3:CD013705. DOI: 10.1002/14651858.CD013705. PMID: 32845525. PMCID: PMC8078202.
Article9. Wang YC, Lee YT, Yang T, Sun JR, Shen CF, Cheng CM. 2020; Current diagnostic tools for coronaviruses-From laboratory diagnosis to POC diagnosis for COVID-19. Bioeng Transl Med. 5:e10177. DOI: 10.1002/btm2.10177. PMID: 32838038. PMCID: PMC7435577.
Article10. FIND. Test directory. https://www.finddx.org/covid-19/test-directory/. Updated on Jan 2022.11. Act on In Vitro Diagnostic Medical Devices. Act no. 16433, Apr. 30, 2019. https://elaw.klri.re.kr/kor_service/lawView.do?hseq=53555&lang=ENG.12. Ministry of Health, Welfare. Infectious Disease Surveillance Yearbook, 2019. GPRN 1-1352159-000048-10. Cheongju: Korea Disease Control and Prevention Agency;2020.13. Lee H, Lee H, Song KH, Kim ES, Park JS, Jung J, et al. 2021; Impact of public health interventions on seasonal influenza activity during the COVID-19 outbreak in Korea. Clin Infect Dis. 73:e132–40. DOI: 10.1093/cid/ciaa672. PMID: 32472687. PMCID: PMC7314207.14. Bioethics And Safety Act. Act No. 16372, Apr. 23, 2019. https://elaw.klri.re.kr/kor_service/lawView.do?lang=ENG&hseq=52559.15. National Institute of Medical Device Safety Information. National Certified Medical Device Regulatory Affairs Specialist 1 - Premarket Approval. Gwangju: Chaoreum;2021.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current Status and Future Perspectives of Renal Denervation
- Medical device clinical trial
- The Current Status and the Perspectives of Nutrition Survey
- How Aware Elderly Subjects are of Medical Device Clinical Trials and Their Adverse Events - A Survery
- Current Status and Perspectives of Cysticercosis and Taeniasis in Japan