Korean Circ J.
2024 Feb;54(2):93-104. 10.4070/kcj.2023.0303.
Feasibility and Effectiveness of a Ring-Type Blood Pressure Measurement Device Compared With 24-Hour Ambulatory Blood Pressure Monitoring Device
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea
- 2Department of Family Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 3Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2552235
- DOI: http://doi.org/10.4070/kcj.2023.0303
Abstract
- Background
s and Objectives: This study aimed to evaluate the applicability and precision of a ring-type cuffless blood pressure (BP) measurement device, CART-I Plus, compared to conventional 24-hour ambulatory BP monitoring (ABPM).
Methods
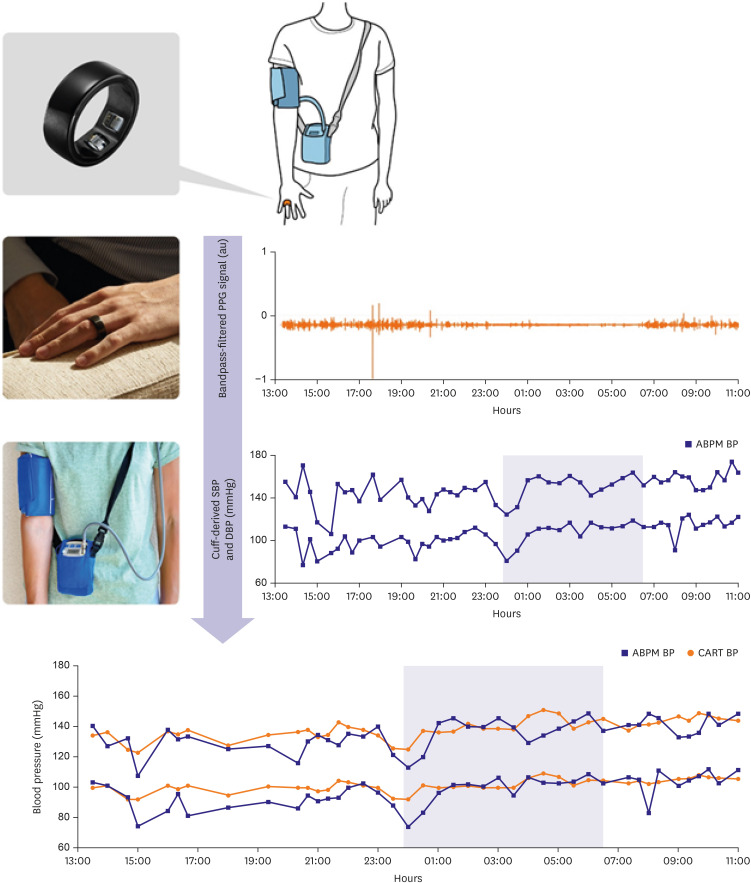
Forty patients were recruited, and 33 participants were included in the final analysis. Each participant wore both CART-I Plus and ABPM devices on the same arm for approximately 24 hours. BP estimation from CART-I Plus, derived from photoplethysmography (PPG) signals, were compared with the corresponding ABPM measurements.
Results
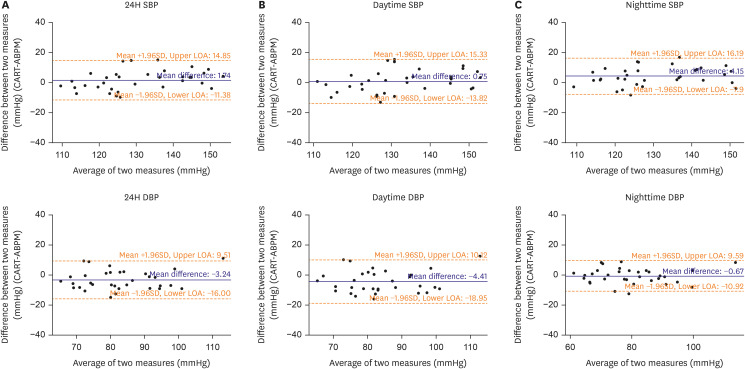
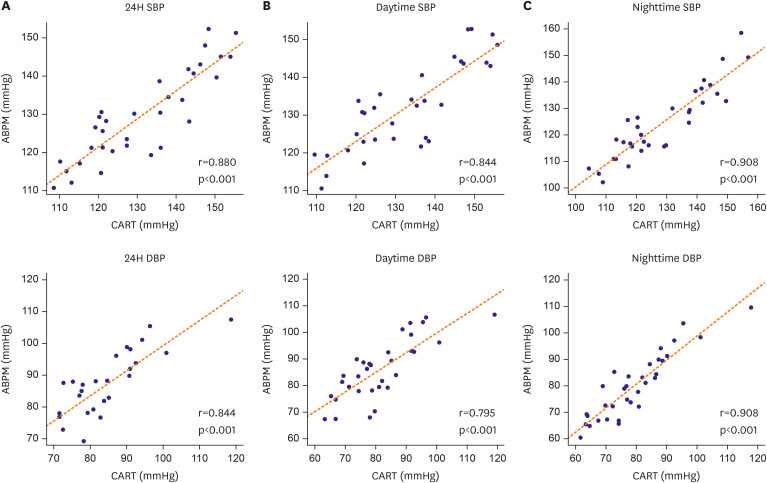
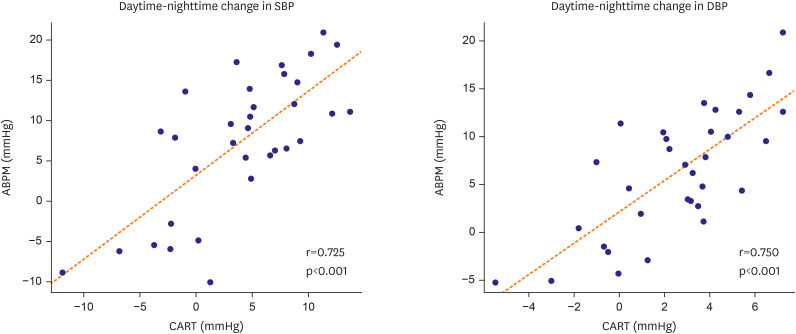
The CART-I Plus recorded systolic blood pressure (SBP)/diastolic blood pressure (DBP) values of 131.4±14.1/81.1±12.0, 132.7±13.9/81.9±11.9, and 128.7±14.6/79.3±12.2 mmHg for 24-hour, daytime, and nighttime periods respectively, compared to ABPM values of 129.7±11.7/84.4±11.2, 131.9±11.6/86.3±11.1, and 124.5±13.6/80.0±12.2 mmHg. Mean differences in SBP/DBP between the two devices were 1.74±6.69/−3.24±6.51 mmHg, 0.75±7.44/−4.41±7.42 mmHg, and 4.15±6.15/−0.67±5.23 mmHg for 24-hour, daytime, and nighttime periods respectively. Strong correlations were also observed between the devices, with r=0.725 and r=0.750 for transitions in SBP and DBP from daytime to nighttime, respectively (both p<0.001).
Conclusions
The CART-I Plus device, with its unique ring-type design, shows promising accuracy in BP estimation and offers a potential avenue for continuous BP monitoring in clinical practice.
Keyword
Figure
Reference
-
1. Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. 2020; 75:285–292. PMID: 31865786.2. Ibrahim B, Jafari R. Cuffless blood pressure monitoring from a wristband with calibration-free algorithms for sensing location based on bio-impedance sensor array and autoencoder. Sci Rep. 2022; 12:319. PMID: 35013376.3. Kario K, Shimada K, Pickering TG. Abnormal nocturnal blood pressure falls in elderly hypertension: clinical significance and determinants. J Cardiovasc Pharmacol. 2003; 41(Suppl 1):S61–S66. PMID: 12688399.4. Kario K, Shimada K. Risers and extreme-dippers of nocturnal blood pressure in hypertension: antihypertensive strategy for nocturnal blood pressure. Clin Exp Hypertens. 2004; 26:177–189. PMID: 15038628.5. Kario K, Pickering TG, Matsuo T, Hoshide S, Schwartz JE, Shimada K. Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension. 2001; 38:852–857. PMID: 11641298.6. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018; 39:3021–3104. PMID: 30165516.7. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018; 71:e13–115. PMID: 29133356.8. Sherwood A, Hill LK, Blumenthal JA, Hinderliter AL. The effects of ambulatory blood pressure monitoring on sleep quality in men and women with hypertension: dipper vs. nondipper and race differences. Am J Hypertens. 2019; 32:54–60. PMID: 30204833.9. Agarwal R, Light RP. The effect of measuring ambulatory blood pressure on nighttime sleep and daytime activity--implications for dipping. Clin J Am Soc Nephrol. 2010; 5:281–285. PMID: 20019118.10. Davies RJ, Jenkins NE, Stradling JR. Effect of measuring ambulatory blood pressure on sleep and on blood pressure during sleep. BMJ. 1994; 308:820–823. PMID: 8167489.11. Stergiou GS, Palatini P, Parati G, et al. 2021 European Society of Hypertension practice guidelines for office and out-of-office blood pressure measurement. J Hypertens. 2021; 39:1293–1302. PMID: 33710173.12. Teng X, Zhang YT. Proceedings of the 25th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (IEEE Cat. No.03CH37439). Piscataway (NJ): Institute of Electrical and Electronics Engineers;2003. p. 3153–3156.13. Elgendi M, Fletcher R, Liang Y, et al. The use of photoplethysmography for assessing hypertension. NPJ Digit Med. 2019; 2:60. PMID: 31388564.14. Stergiou GS, Mukkamala R, Avolio A, et al. Cuffless blood pressure measuring devices: review and statement by the European Society of Hypertension Working Group on Blood Pressure Monitoring and Cardiovascular Variability. J Hypertens. 2022; 40:1449–1460. PMID: 35708294.15. El-Hajj C, Kyriacou PA. A review of machine learning techniques in photoplethysmography for the non-invasive cuff-less measurement of blood pressure. Biomed Signal Process Control. 2020; 58:101870.16. Stergiou GS, Avolio AP, Palatini P, et al. European Society of Hypertension recommendations for the validation of cuffless blood pressure measuring devices: European Society of Hypertension Working Group on Blood Pressure Monitoring and Cardiovascular Variability. J Hypertens. 2023; 41:2074–2087. PMID: 37303198.17. Joung J, Jung CW, Lee HC, et al. Continuous cuffless blood pressure monitoring using photoplethysmography-based PPG2BP-net for high intrasubject blood pressure variations. Sci Rep. 2023; 13:8605. PMID: 37244974.18. Charmoy A, Würzner G, Ruffieux C, et al. Reactive rise in blood pressure upon cuff inflation: cuff inflation at the arm causes a greater rise in pressure than at the wrist in hypertensive patients. Blood Press Monit. 2007; 12:275–280. PMID: 17890965.19. International Organization for Standardization (ISO). ISO 81060-2:2018 Non-invasive sphygmomanometers—Part 2: Clinical investigation of intermittent automated measurement type. Geneva: International Organization for Standardization;2018.20. Islam SM, Cartledge S, Karmakar C, et al. Validation and acceptability of a cuffless wrist-worn wearable blood pressure monitoring device among users and health care professionals: mixed methods study. JMIR Mhealth Uhealth. 2019; 7:e14706. PMID: 31628788.21. Nachman D, Gilan A, Goldstein N, et al. Twenty-four-hour ambulatory blood pressure measurement using a novel noninvasive, cuffless, wireless device. Am J Hypertens. 2021; 34:1171–1180. PMID: 34143867.22. Falter M, Scherrenberg M, Driesen K, et al. Smartwatch-based blood pressure measurement demonstrates insufficient accuracy. Front Cardiovasc Med. 2022; 9:958212. PMID: 35898281.23. Sola J, Cortes M, Perruchoud D, et al. Guidance for the interpretation of continual cuffless blood pressure data for the diagnosis and management of hypertension. Front Med Technol. 2022; 4:899143. PMID: 35655524.24. Tan I, Gnanenthiran SR, Chan J, et al. Evaluation of the ability of a commercially available cuffless wearable device to track blood pressure changes. J Hypertens. 2023; 41:1003–1010. PMID: 37016925.25. Park SW. Prospective, Single-center, Single group, Pivotal clinical trial to evaluate the blood pressure accuracy of ‘CART-I plus’ compared to the reference blood pressure reading with an auscultatory sphygmomanometer. J Korean Med Sci. 2024; [Epub ahead of print].26. Nwankwo T, Coleman King SM, Ostchega Y, et al. Comparison of 3 devices for 24-hour ambulatory blood pressure monitoring in a nonclinical environment through a randomized trial. Am J Hypertens. 2020; 33:1021–1029. PMID: 32701144.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Study of 24 Hour Ambulatory Blood Pressure Monitoring in Acute Stroke Patients
- Comparison of the ambulatory blood pressure with the clinical blood pressure and electrocardiographic left ventricular hypertrophy
- A representative value for 24-hour monitored ambulatory blood pressure
- Noninvasive ambulatory blood pressure monitoring in 22 healthy normotensive young adolescents
- Comparison of Blood Pressure Control and Left Ventricular Hypertrophy in Patients on Continuous Ambulatory Peritoneal Dialysis (CAPD) and Automated Peritoneal Dialysis (APD)