Ann Lab Med.
2023 May;43(3):307-309. 10.3343/alm.2023.43.3.307.
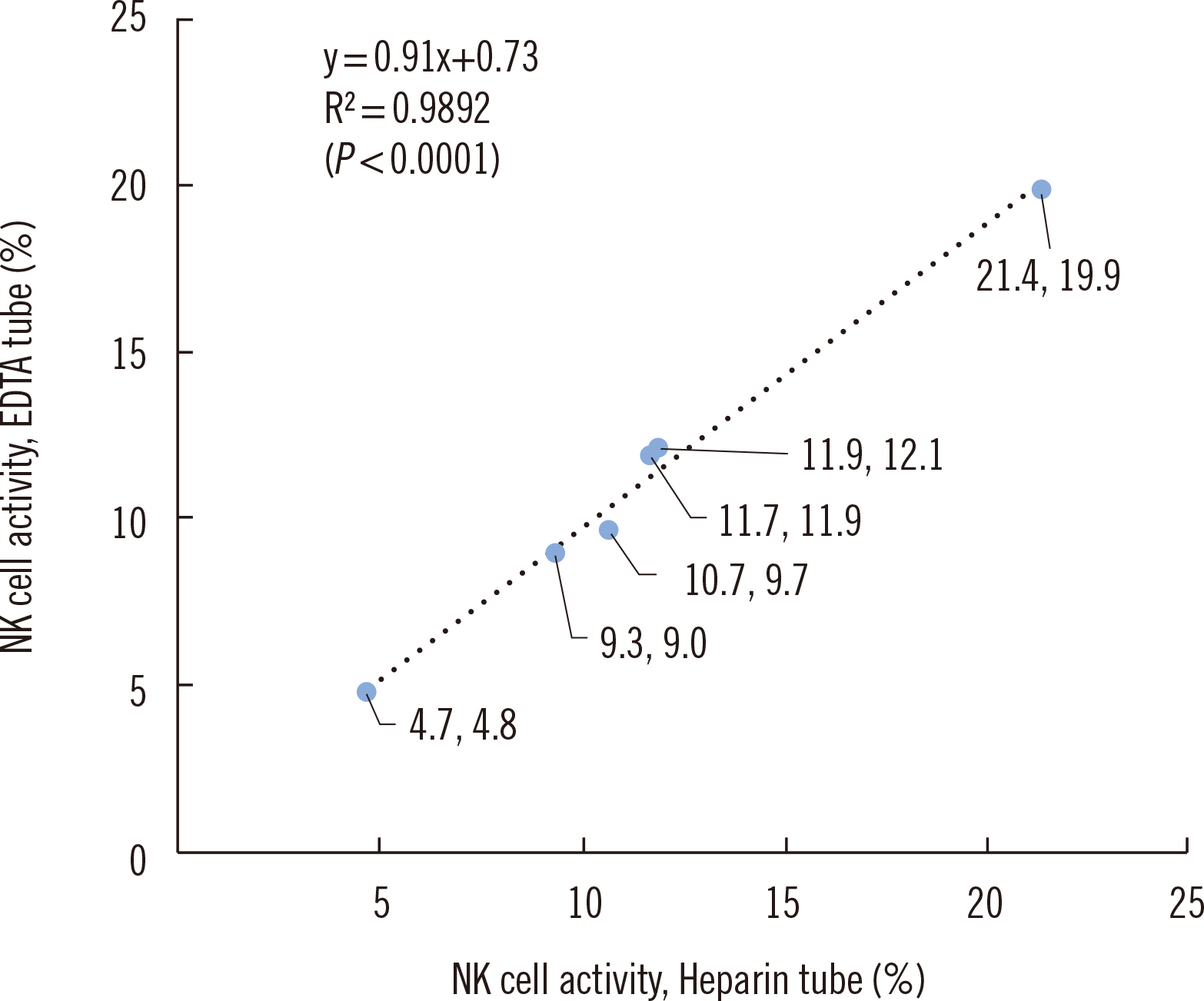
Suitability of EDTA-anticoagulated Blood for Natural Killer Cell Activity Testing Using Flow Cytometry
- Affiliations
-
- 1Department of Laboratory Medicine, Asan Medical Center, Seoul, Korea
- 2Department of Laboratory Medicine, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2551717
- DOI: http://doi.org/10.3343/alm.2023.43.3.307
Figure
Reference
-
1. Henter JI, Horne A, Aricó M, Egeler RM, Filipovich AH, Imashuku S, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007; 48:124–31. DOI: 10.1002/pbc.21039. PMID: 16937360.
Article2. Cichocki F. 2021; Impact of Adaptive NK Cells in Donor Lymphocyte Infusions for the Treatment of Hematological Malignancies. Transplant Cell Ther. 27:101–2. DOI: 10.1016/j.jtct.2021.01.012. PMID: 33781543.
Article3. Piriou L, Chilmonczyk S, Genetet N, Albina E. 2000; Design of a flow cytometric assay for the determination of natural killer and cytotoxic T-lymphocyte activity in human and in different animal species. Cytometry. 41:289–97. DOI: 10.1002/1097-0320(20001201)41:4<289::AID-CYTO7>3.0.CO;2-5. PMID: 11084614.
Article4. Vizler C, Nagy T, Kusz E, Glavinas H, Duda E. 2002; Flow cytometric cytotoxicity assay for measuring mammalian and avian NK cell activity. Cytometry. 47:158–62. DOI: 10.1002/cyto.10066. PMID: 11891720.
Article5. Bromelow KV, Galea-Lauri J, O'Brien ME, Souberbielle BE. 1998; A highly sensitive whole blood natural killer cell assay. J Immunol Methods. 217:177–84. DOI: 10.1016/S0022-1759(98)00109-4. PMID: 9776587.
Article6. Koh SK, Park J, Kim SE, Lim Y, Phan MT, Kim J, et al. 2022; Natural Killer Cell Expansion and Cytotoxicity Differ Depending on the Culture Medium Used. Ann Lab Med. 42:638–49. DOI: 10.3343/alm.2022.42.6.638. PMID: 35765872. PMCID: PMC9277036.
Article7. Chung HJ, Park CJ, Lim JH, Jang S, Chi HS, Im HJ, et al. 2010; Establishment of a reference interval for natural killer cell activity through flow cytometry and its clinical application in the diagnosis of hemophagocytic lymphohistiocytosis. Int J Lab Hematol. 32:239–47. DOI: 10.1111/j.1751-553X.2009.01177.x. PMID: 19614711.
Article8. Son BK, Roberts RL, Ank BJ, Stiehm ER. 1996; Effects of anticoagulant, serum, and temperature on the natural killer activity of human peripheral blood mononuclear cells stored overnight. Clin Diagn Lab Immunol. 3:260–4. DOI: 10.1128/cdli.3.3.260-264.1996. PMID: 8705665. PMCID: PMC170325.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cytotoxic activity and subset populations of peripheral blood natural killer cells in patients with chronic pain
- A Study of Natural Killer Cell Activities , T Cells and T Cell Subsets in Vitiligo
- Elevated natural killer cell levels and autoimmunity synergistically decrease uterine blood flow during early pregnancy
- Clinical significance of cell-mediated immunity and natural killer cell activity related with septic complication in panperitonitis patients
- The Effect of Tumor Removal and Administration of OK432 on the Splenic Natural Killer Cell Activity in the Subcutaneous Tumor Bearing Rats