Ann Lab Med.
2023 Jan;43(1):96-99. 10.3343/alm.2023.43.1.96.
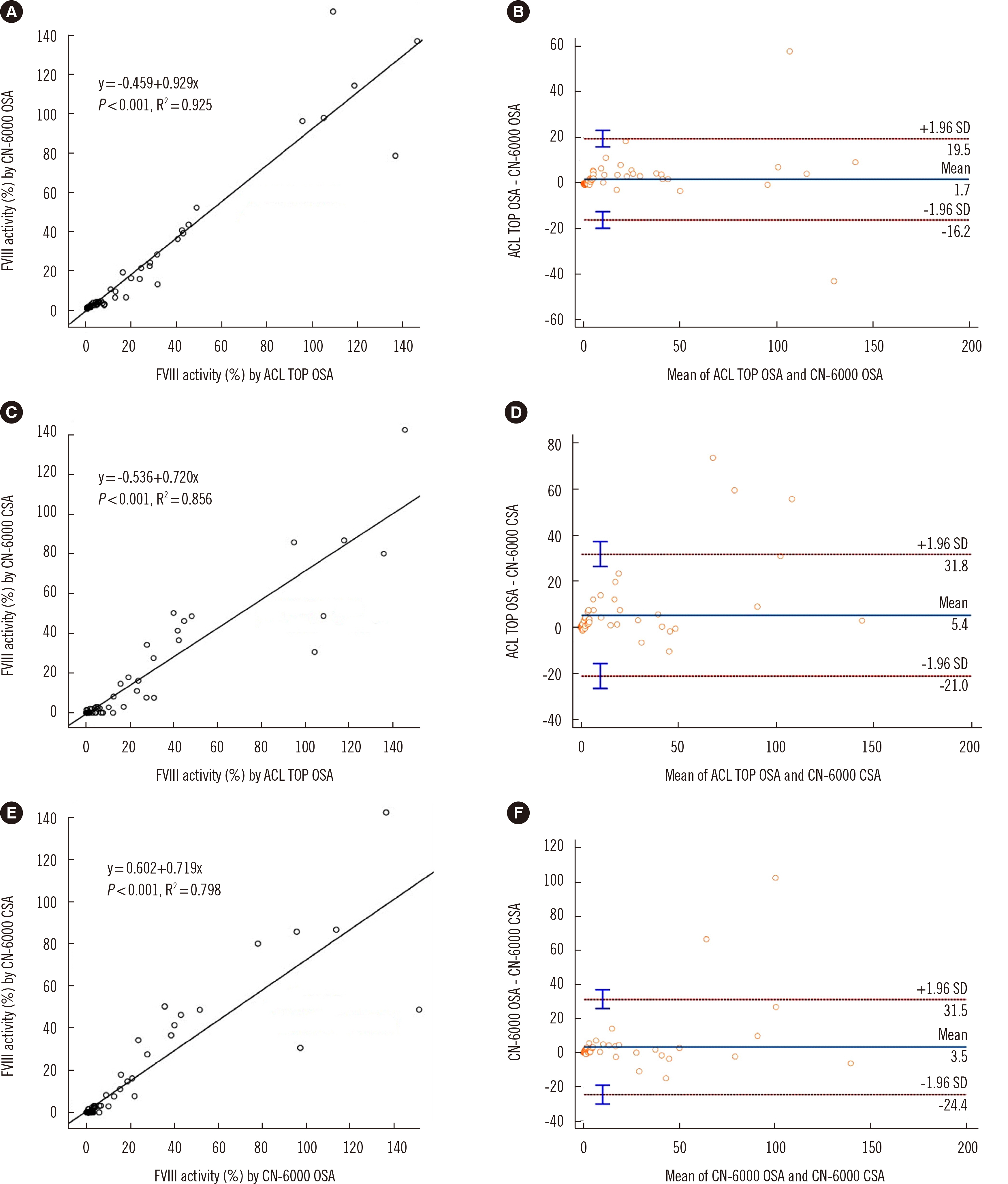
Verification and Comparison of Chromogenic Factor VIII Activity Assays in Patients With Hemophilia Treated With and Without Emicizumab
- Affiliations
-
- 1Department of Laboratory Medicine, Kyung Hee University School of Medicine and Kyung Hee University Hospital at Gangdong, Seoul, Korea
- 2Department of Pediatrics, Kyung Hee University School of Medicine and Kyung Hee University Hospital at Gangdong, Seoul, Korea
- KMID: 2551609
- DOI: http://doi.org/10.3343/alm.2023.43.1.96
Figure
Reference
-
1. Shima M, Hanabusa H, Taki M, Matsushita T, Sato T, Fukutake K, et al. 2016; Factor VIII-mimetic function of humanized bispecific antibody in hemophilia A. N Engl J Med. 374:2044–53. DOI: 10.1056/NEJMoa1511769. PMID: 27223146.
Article2. Bowyer A, Kitchen S, Maclean R. 2020; Effects of emicizumab on APTT, one-stage and chromogenic assays of factor VIII in artificially spiked plasma and in samples from haemophilia A patients with inhibitors. Haemophilia. 26:536–42. DOI: 10.1111/hae.13990. PMID: 32249990.
Article3. Administration FaD. HEMLIBRA (emicizumab-kxwh) injection for subcutaneous use, prescribing information. In: Administration FaD, ed. Initial U.S.;2017.4. Jenkins PV, Bowyer A, Burgess C, Gray E, Kitchen S, Murphy P, et al. 2020; Laboratory coagulation tests and emicizumab treatment A United Kingdom Haemophilia Centre Doctors' Organisation guideline. Haemophilia. 26:151–5. DOI: 10.1111/hae.13903. PMID: 31859415.
Article5. Nougier C, Jeanpierre E, Ternisien C, Proulle V, Hezard N, Pouplard C, et al. 2020; Emicizumab treatment: impact on coagulation tests and biological monitoring of haemostasis according to clinical situations (BIMHO group proposals). Eur J Haematol. 105:675–81. DOI: 10.1111/ejh.13490. PMID: 32668090.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An Autoplex Treatment in a Hemophilia A Patient with High Titer of Anticoagulant FVIII Antibody
- Factor VIII Gene Inversions in Korean Patients with Severe Hemophilia A and its Application to Carrier Detection
- Anesthetic Management of Open Heart Surgery in a Patient with Hemophilia A: A case report
- Two patients with acquired hemophilia successfully treated with combination therapy including therapeutic plasmapheresis
- Genetic Risk Factors of Hemophilia A