Intest Res.
2024 Jan;22(1):92-103. 10.5217/ir.2023.00105.
Association of colonic metaplasia of goblet cells and endoscopic phenotypes of the J pouch in patients with ulcerative colitis: a retrospective pilot study
- Affiliations
-
- 1Department of Gastroenterology, Institute of Medicine, Faculty of Medicine, University of Tsukuba, Tsukuba, Japan
- 2Department of Gastrointestinal and Hepato-Biliary-Pancreatic Surgery, Institute of Medicine, Faculty of Medicine, University of Tsukuba, Tsukuba, Japan
- 3Department of Pathology, Institute of Medicine, Faculty of Medicine, University of Tsukuba, Tsukuba, Japan
- KMID: 2551282
- DOI: http://doi.org/10.5217/ir.2023.00105
Abstract
- Background/Aims
Mucosal adaptation of the ileum toward colonic epithelium has been reported in pouchitis in ulcerative colitis (UC); however, the clinical characteristics, endoscopic findings, and outcomes in patients with pouchitis with ileal mucosal adaptation are poorly understood.
Methods
This was a single-center retrospective study comprising UC patients treated by proctocolectomy with ileal pouch-anal anastomosis who had undergone pouchoscopy at the University of Tsukuba Hospital between 2005 and 2022. Endoscopic phenotypes were evaluated according to the Chicago classification. High-iron diamine staining (HID) was performed to identify sulfomucin (colon-type mucin)-producing goblet cells (GCs) in pouch biopsies. We compared clinical data between patients with (high HID group) and without > 10% sulfomucin-producing GCs in at least one biopsy (low HID group).
Results
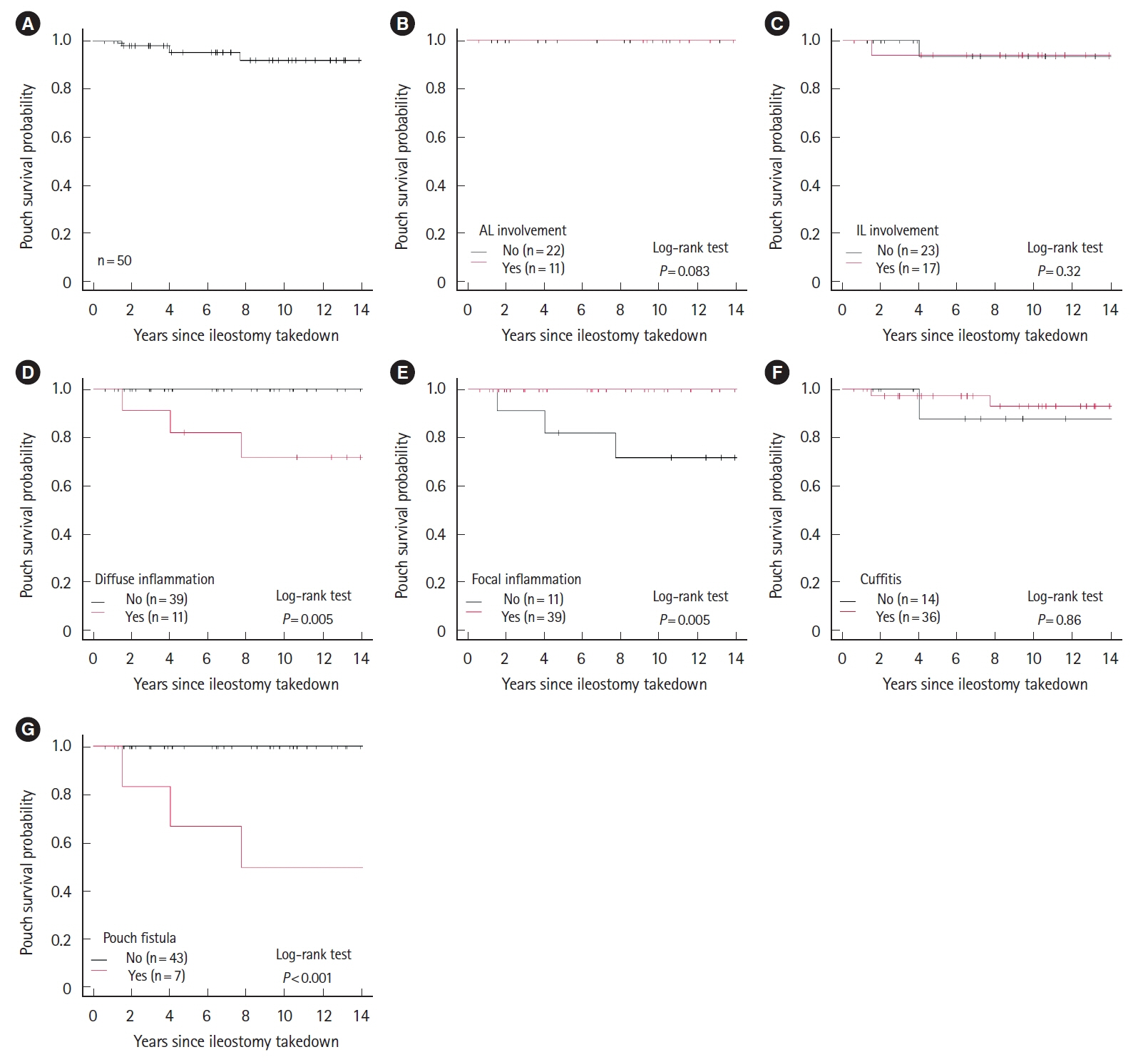
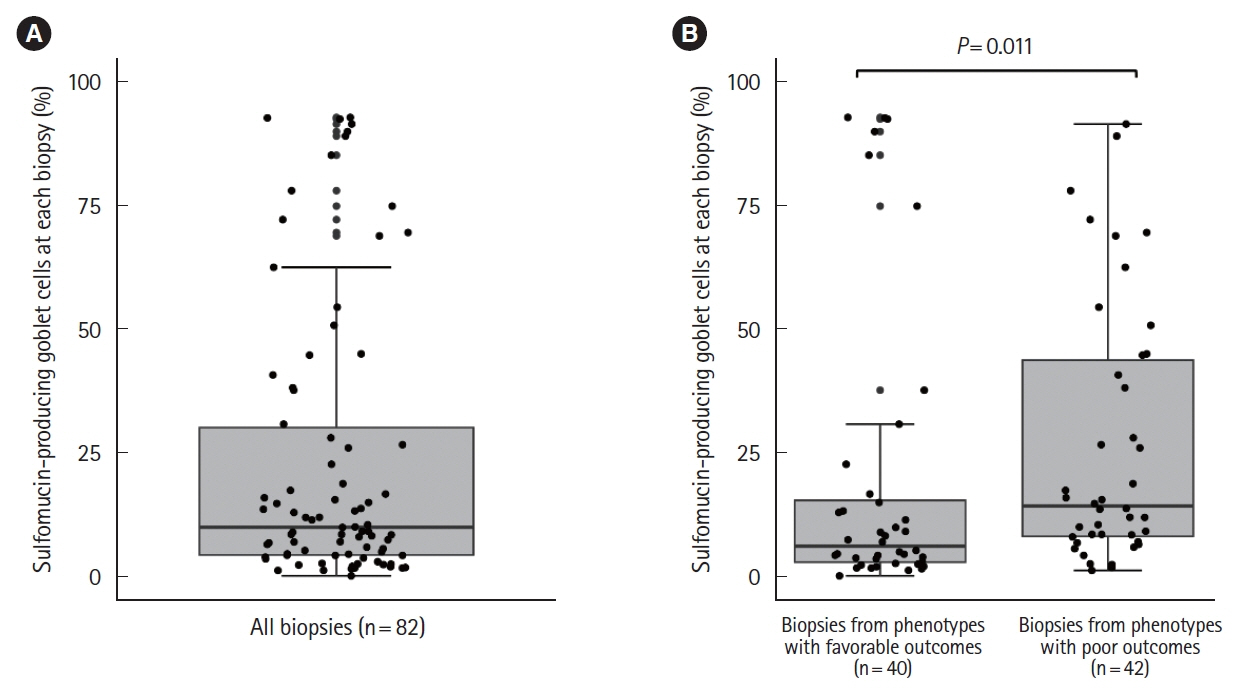
We reviewed 390 endoscopic examination reports from 50 patients. Focal inflammation was the most common phenotype (78%). Five patients (10%) required diverting ileostomy. Diffuse inflammation and fistula were significant risk factors for diverting ileostomy. The median proportion of sulfomucin-producing GCs on histological analysis of 82 pouch biopsies from 23 patients was 9.9% (range, 0%–93%). The duration of disease was significantly greater in the high HID group compared to the low HID group. The median percentage of sulfomucin-producing GCs was significantly higher in patients with diffuse inflammation or fistula compared to other endoscopic phenotypes (14% vs. 6.0%, P= 0.011).
Conclusions
Greater proportions of sulfomucin-producing GCs were observed in endoscopic phenotypes associated with poor outcomes in UC, indicating patients with pouchitis showing colonic metaplasia of GCs may benefit from early interventions.
Figure
Reference
-
1. Fumery M, Singh S, Dulai PS, Gower-Rousseau C, Peyrin-Biroulet L, Sandborn WJ. Natural history of adult ulcerative colitis in population-based cohorts: a systematic review. Clin Gastroenterol Hepatol. 2018; 16:343–356.
Article2. Akiyama S, Rai V, Rubin DT. Pouchitis in inflammatory bowel disease: a review of diagnosis, prognosis, and treatment. Intest Res. 2021; 19:1–11.
Article3. Kayal M, Plietz M, Rizvi A, et al. Inflammatory pouch conditions are common after ileal pouch anal anastomosis in ulcerative colitis patients. Inflamm Bowel Dis. 2020; 26:1079–1086.
Article4. Barnes EL, Herfarth HH, Kappelman MD, et al. Incidence, risk factors, and outcomes of pouchitis and pouch-related complications in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2021; 19:1583–1591.
Article5. Akiyama S, Dyer EC, Rubin DT. Diagnostic and management considerations for the IPAA with Crohn’s disease-like features. Dis Colon Rectum. 2022; 65(S1):S77–S84.
Article6. Ricardo AP, Kayal M, Plietz MC, et al. Predictors of pouch failure: a tertiary care inflammatory bowel disease centre experience. Colorectal Dis. 2023; 25:1469–1478.
Article7. Shen B, Kochhar GS, Kariv R, et al. Diagnosis and classification of ileal pouch disorders: consensus guidelines from the International Ileal Pouch Consortium. Lancet Gastroenterol Hepatol. 2021; 6:826–849.
Article8. Shen B, Kochhar GS, Rubin DT, et al. Treatment of pouchitis, Crohn’s disease, cuffitis, and other inflammatory disorders of the pouch: consensus guidelines from the International Ileal Pouch Consortium. Lancet Gastroenterol Hepatol. 2022; 7:69–95.9. Zhu H, Wu XR, Queener E, Kiran RP, Remzi FH, Shen B. Clinical value of surveillance pouchoscopy in asymptomatic ileal pouch patients with underlying inflammatory bowel disease. Surg Endosc. 2013; 27:4325–4332.
Article10. Kayal M, Plietz M, Radcliffe M, et al. Endoscopic activity in asymptomatic patients with an ileal pouch is associated with an increased risk of pouchitis. Aliment Pharmacol Ther. 2019; 50:1189–1194.
Article11. Akiyama S, Ollech JE, Rai V, et al. Endoscopic phenotype of the J pouch in patients with inflammatory bowel disease: a new classification for pouch outcomes. Clin Gastroenterol Hepatol. 2022; 20:293–302.
Article12. Xu W, Wang Y, Hua Z, et al. Risk factors and quality of life in patients with diffuse pouchitis after ileal pouch anal anastomosis according to the Chicago classification for J pouch: a retrospective multicenter cohort study in China. J Gastrointest Surg. 2023; 27:766–776.
Article13. Akiyama S, Rodriguez TG, Rubin DT. Stratifying risks of ileal pouch outcomes. Clin Gastroenterol Hepatol. 2022; 20:1620–1621.14. Kayal M, Ungaro RC, Colombel JF. The Chicago classification of pouchitis: an important step toward a needed consensus. Clin Gastroenterol Hepatol. 2022; 20:281–282.15. Deplancke B, Gaskins HR. Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Am J Clin Nutr. 2001; 73:1131S–1141S.16. Akiyama S, Mochizuki W, Nibe Y, et al. CCN3 expression marks a sulfomucin-nonproducing unique subset of colonic goblet cells in mice. Acta Histochem Cytochem. 2017; 50:159–168.17. Croix JA, Carbonero F, Nava GM, Russell M, Greenberg E, Gaskins HR. On the relationship between sialomucin and sulfomucin expression and hydrogenotrophic microbes in the human colonic mucosa. PLoS One. 2011; 6:e24447.
Article18. Filipe MI, Fenger C. Histochemical characteristics of mucins in the small intestine: a comparative study of normal mucosa, benign epithelial tumours and carcinoma. Histochem J. 1979; 11:277–287.
Article19. Apel R, Cohen Z, Andrews CW Jr, McLeod R, Steinhart H, Odze RD. Prospective evaluation of early morphological changes in pelvic ileal pouches. Gastroenterology. 1994; 107:435–443.
Article20. Garcia-Armengol J, Hinojosa J, Lledo S, Roig JV, Garcia-Granero E, Martinez B. Prospective study of morphologic and functional changes with time in the mucosa of the ileoanal pouch: functional appraisal using transmucosal potential differences. Dis Colon Rectum. 1998; 41:846–853.
Article21. Chadha K, Mukherjee B, Subramanya H, Singh KV. Restorative proctocolectomy with ileo-anal reservoir, a histopathological, histochemical, and electron microscopic study. Med J Armed Forces India. 2003; 59:306–309.
Article22. Bambury N, Coffey JC, Burke J, Redmond HP, Kirwan WO. Sulphomucin expression in ileal pouches: emerging differences between ulcerative colitis and familial adenomatous polyposis pouches. Dis Colon Rectum. 2008; 51:561–567.
Article23. Sandborn WJ, Tremaine WJ, Batts KP, Pemberton JH, Phillips SF. Pouchitis after ileal pouch-anal anastomosis: a Pouchitis Disease Activity Index. Mayo Clin Proc. 1994; 69:409–415.
Article24. Shen B, Achkar JP, Connor JT, et al. Modified Pouchitis Disease Activity Index: a simplified approach to the diagnosis of pouchitis. Dis Colon Rectum. 2003; 46:748–753.25. Shen B, Remzi FH, Lavery IC, Lashner BA, Fazio VW. A proposed classification of ileal pouch disorders and associated complications after restorative proctocolectomy. Clin Gastroenterol Hepatol. 2008; 6:145–158.
Article26. Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006; 55:749–753.
Article27. Spicer SS. Diamine methods for differentialing mucosubstances histochemically. J Histochem Cytochem. 1965; 13:211–234.28. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013; 48:452–458.
Article29. Akiyama S, Ollech JE, Traboulsi C, et al. Histopathology of colectomy specimens predicts endoscopic pouch phenotype in patients with ulcerative colitis. Dig Dis Sci. 2022; 67:4020–4031.
Article30. Lavryk OA, Stocchi L, Hull TL, et al. Impact of preoperative duration of ulcerative colitis on long-term outcomes of restorative proctocolectomy. Int J Colorectal Dis. 2020; 35:41–49.
Article31. Ohge H, Furne JK, Springfield J, Rothenberger DA, Madoff RD, Levitt MD. Association between fecal hydrogen sulfide production and pouchitis. Dis Colon Rectum. 2005; 48:469–475.
Article32. Coffey JC, Bennett MW, Wang JH, et al. Upregulation of FasFas-L (CD95/CD95L)-mediated epithelial apoptosis: a putative role in pouchitis? J Surg Res. 2001; 98:27–32.
Article33. Kmiot WA, Youngs D, Tudor R, Thompson H, Keighley MR. Mucosal morphology, cell proliferation and faecal bacteriology in acute pouchitis. Br J Surg. 1993; 80:1445–1449.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Alternation of mucin structure and lectin binding in the mucosa of ulcerative colitis
- Malignant change of chronic ulcerative colitis : report of a case
- Fine Structure of Goblet Cell Regeneration on Experimental Colitis Induced by Dextran Sulfate Sodium
- The prevalence of pouch fistulas in ulcerative colitis following restorative proctocolectomy: a systematic review and meta-analysis
- A Case of Benign Colonic Stricture Treated by Therapeutic Balloon Dilatation in Ulcerative Colitis