Diabetes Metab J.
2024 Jan;48(1):19-36. 10.4093/dmj.2023.0110.
Primordial Drivers of Diabetes Heart Disease: Comprehensive Insights into Insulin Resistance
- Affiliations
-
- 1Department of Cardiovascular, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 2Department of Cardiovascular, National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion, Tianjin, China
- 3Institute of Gerontology, Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- KMID: 2551259
- DOI: http://doi.org/10.4093/dmj.2023.0110
Abstract
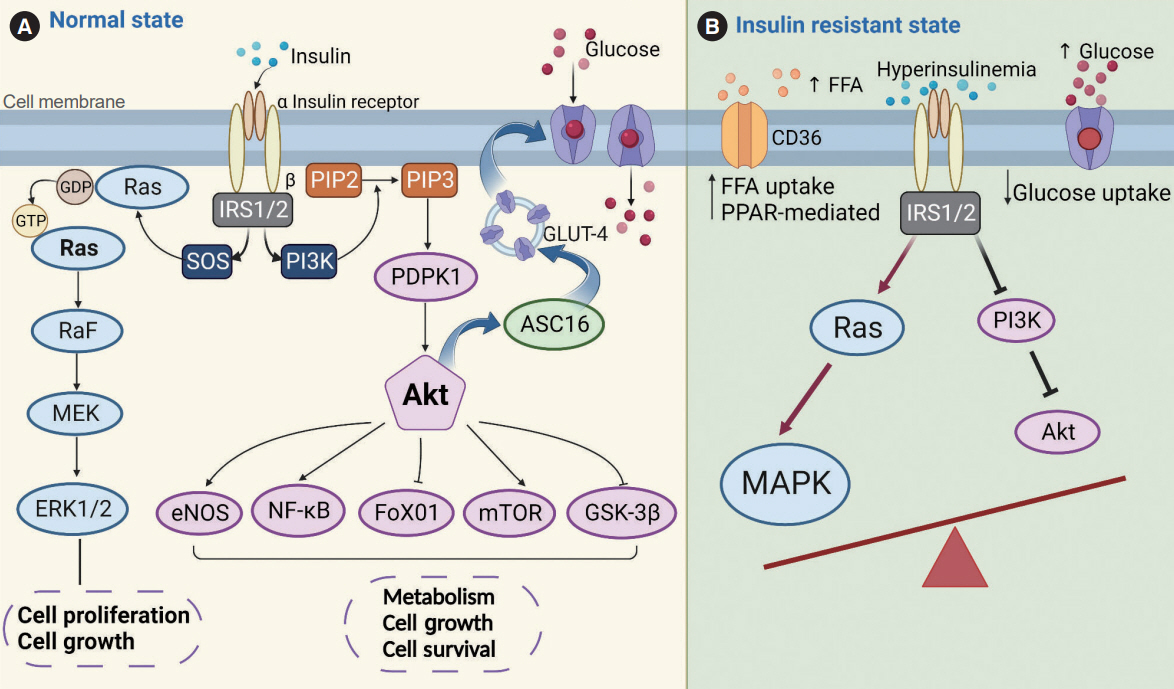
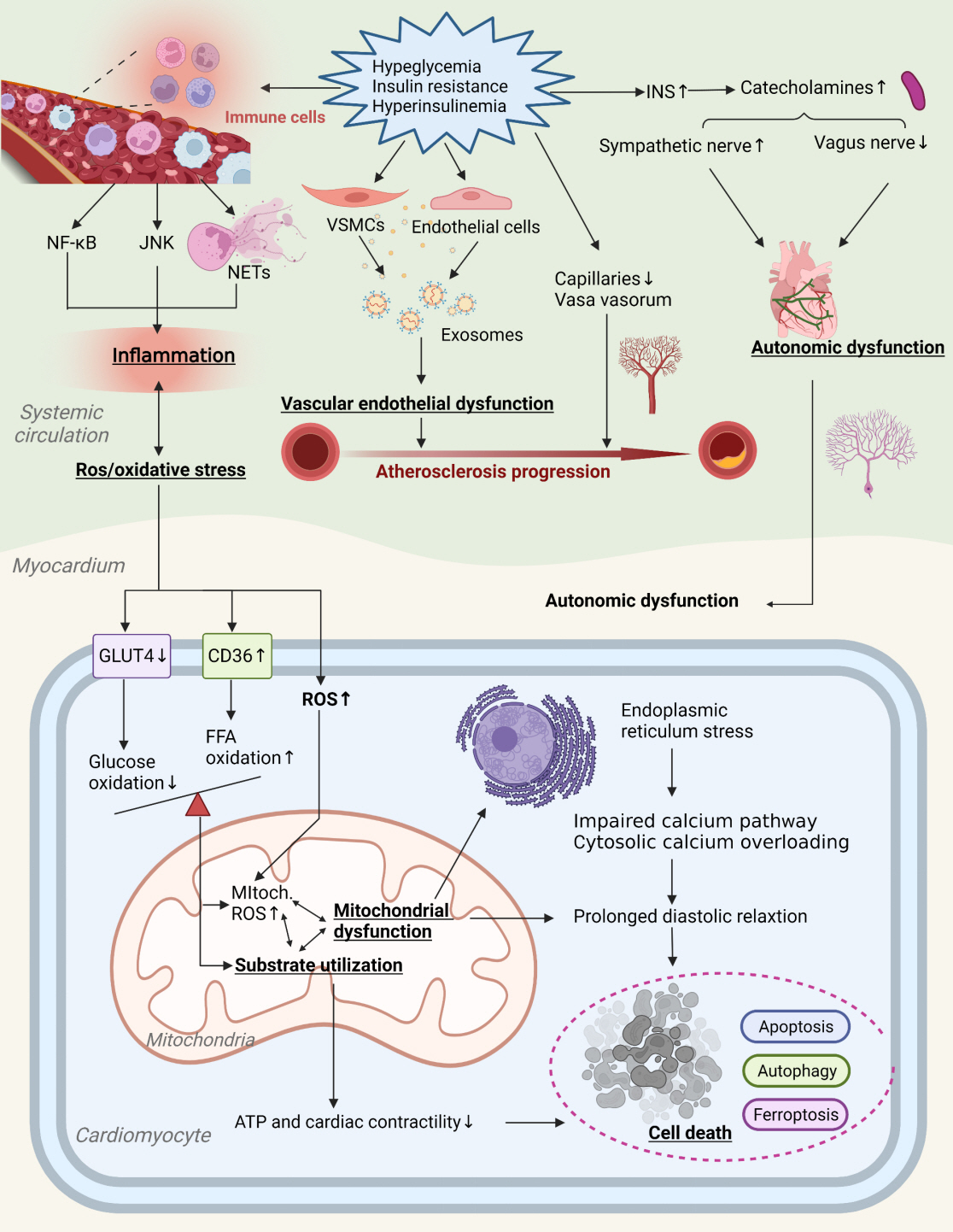
- Insulin resistance has been regarded as a hallmark of diabetes heart disease (DHD). Numerous studies have shown that insulin resistance can affect blood circulation and myocardium, which indirectly cause cardiac hypertrophy and ventricular remodeling, participating in the pathogenesis of DHD. Meanwhile, hyperinsulinemia, hyperglycemia, and hyperlipidemia associated with insulin resistance can directly impair the metabolism and function of the heart. Targeting insulin resistance is a potential therapeutic strategy for the prevention of DHD. Currently, the role of insulin resistance in the pathogenic development of DHD is still under active research, as the pathological roles involved are complex and not yet fully understood, and the related therapeutic approaches are not well developed. In this review, we describe insulin resistance and add recent advances in the major pathological and physiological changes and underlying mechanisms by which insulin resistance leads to myocardial remodeling and dysfunction in the diabetic heart, including exosomal dysfunction, ferroptosis, and epigenetic factors. In addition, we discuss potential therapeutic approaches to improve insulin resistance and accelerate the development of cardiovascular protection drugs.
Keyword
Figure
Reference
-
1. Ritchie RH, Abel ED. Basic mechanisms of diabetic heart disease. Circ Res. 2020; 126:1501–25.2. Joseph P, Leong D, McKee M, Anand SS, Schwalm JD, Teo K, et al. Reducing the global burden of cardiovascular disease. Part 1: the epidemiology and risk factors. Circ Res. 2017; 121:677–94.3. Adeva-Andany MM, Martinez-Rodriguez J, Gonzalez-Lucan M, Fernandez-Fernandez C, Castro-Quintela E. Insulin resistance is a cardiovascular risk factor in humans. Diabetes Metab Syndr. 2019; 13:1449–55.4. Laakso M. Is insulin resistance a feature of or a primary risk factor for cardiovascular disease? Curr Diab Rep. 2015; 15:105.5. Robins SJ, Rubins HB, Faas FH, Schaefer EJ, Elam MB, Anderson JW, et al. Insulin resistance and cardiovascular events with low HDL cholesterol: the Veterans Affairs HDL Intervention Trial (VA-HIT). Diabetes Care. 2003; 26:1513–7.6. Hanley AJ, Williams K, Stern MP, Haffner SM. Homeostasis model assessment of insulin resistance in relation to the incidence of cardiovascular disease: the San Antonio Heart Study. Diabetes Care. 2002; 25:1177–84.7. Saltiel AR. Insulin signaling in health and disease. J Clin Invest. 2021; 131:e142241.8. Ye H, He Y, Zheng C, Wang F, Yang M, Lin J, et al. Type 2 diabetes complicated with heart failure: research on therapeutic mechanism and potential drug development based on insulin signaling pathway. Front Pharmacol. 2022; 13:816588.9. Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev. 2018; 98:2133–23.10. Plotnikov A, Zehorai E, Procaccia S, Seger R. The MAPK cascades: signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim Biophys Acta. 2011; 1813:1619–33.11. Harmancey R, Haight DL, Watts KA, Taegtmeyer H. Chronic hyperinsulinemia causes selective insulin resistance and downregulates uncoupling protein 3 (UCP3) through the activation of sterol regulatory element-binding protein (SREBP)-1 transcription factor in the mouse heart. J Biol Chem. 2015; 290:30947–61.12. Wang CC, Gurevich I, Draznin B. Insulin affects vascular smooth muscle cell phenotype and migration via distinct signaling pathways. Diabetes. 2003; 52:2562–9.13. Paneni F, Costantino S, Cosentino F. Insulin resistance, diabetes, and cardiovascular risk. Curr Atheroscler Rep. 2014; 16:419.14. Gargiulo P, Perrone-Filardi P. “Heart failure, whole-body insulin resistance and myocardial insulin resistance: an intriguing puzzle”. J Nucl Cardiol. 2018; 25:177–80.15. Wang LY, Chen C. Energy metabolism homeostasis in cardiovascular diseases. J Geriatr Cardiol. 2021; 18:1044–57.16. Harmsen JF, Wefers J, Doligkeit D, Schlangen L, Dautzenberg B, Rense P, et al. The influence of bright and dim light on substrate metabolism, energy expenditure and thermoregulation in insulin-resistant individuals depends on time of day. Diabetologia. 2022; 65:721–32.17. Han B, Wang J, Wu J, Yan F, Wang Y, Li J. High glucose-induced upregulation of CD36 promotes inflammation stress via NF-κB in H9c2 cells. Mol Med Rep. 2021; 24:764.18. Shu H, Peng Y, Hang W, Nie J, Zhou N, Wang DW. The role of CD36 in cardiovascular disease. Cardiovasc Res. 2022; 118:115–29.19. Song F, Mao YJ, Hu Y, Zhao SS, Wang R, Wu WY, et al. Acacetin attenuates diabetes-induced cardiomyopathy by inhibiting oxidative stress and energy metabolism via PPAR-α/AMPK pathway. Eur J Pharmacol. 2022; 922:174916.20. Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuniga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018; 17:122.21. Karwi QG, Wagg CS, Altamimi TR, Uddin GM, Ho KL, Darwesh AM, et al. Insulin directly stimulates mitochondrial glucose oxidation in the heart. Cardiovasc Diabetol. 2020; 19:207.22. Longo M, Scappaticcio L, Cirillo P, Maio A, Carotenuto R, Maiorino MI, et al. Glycemic control and the heart: the tale of diabetic cardiomyopathy continues. Biomolecules. 2022; 12:272.23. Salvatore T, Pafundi PC, Galiero R, Albanese G, Di Martino A, Caturano A, et al. The diabetic cardiomyopathy: the contributing pathophysiological mechanisms. Front Med (Lausanne). 2021; 8:695792.24. Daniels MC, McClain DA, Crook ED. Transcriptional regulation of transforming growth factor β1 by glucose: investigation into the role of the hexosamine biosynthesis pathway. Am J Med Sci. 2020; 359:79–83.25. Lopaschuk GD, Karwi QG, Ho KL, Pherwani S, Ketema EB. Ketone metabolism in the failing heart. Biochim Biophys Acta Mol Cell Biol Lipids. 2020; 1865:158813.26. Roberts MN, Wallace MA, Tomilov AA, Zhou Z, Marcotte GR, Tran D, et al. A ketogenic diet extends longevity and healthspan in adult mice. Cell Metab. 2018; 27:1156.27. Newman JC, Covarrubias AJ, Zhao M, Yu X, Gut P, Ng CP, et al. Ketogenic diet reduces midlife mortality and improves memory in aging mice. Cell Metab. 2017; 26:547–57.28. Garcia E, Shalaurova I, Matyus SP, Oskardmay DN, Otvos JD, Dullaart RP, et al. Ketone bodies are mildly elevated in subjects with type 2 diabetes mellitus and are inversely associated with insulin resistance as measured by the lipoprotein insulin resistance index. J Clin Med. 2020; 9:321.29. Aroor AR, Mandavia CH, Sowers JR. Insulin resistance and heart failure: molecular mechanisms. Heart Fail Clin. 2012; 8:609–17.30. Trost SU, Belke DD, Bluhm WF, Meyer M, Swanson E, Dillmann WH. Overexpression of the sarcoplasmic reticulum Ca(2+)-ATPase improves myocardial contractility in diabetic cardiomyopathy. Diabetes. 2002; 51:1166–71.31. Quan C, Du Q, Li M, Wang R, Ouyang Q, Su S, et al. A PKBSPEG signaling nexus links insulin resistance with diabetic cardiomyopathy by regulating calcium homeostasis. Nat Commun. 2020; 11:2186.32. Roe ND, He EY, Wu Z, Ren J. Folic acid reverses nitric oxide synthase uncoupling and prevents cardiac dysfunction in insulin resistance: role of Ca2+/calmodulin-activated protein kinase II. Free Radic Biol Med. 2013; 65:234–43.33. Qin L, Zang M, Xu Y, Zhao R, Wang Y, Mi Y, et al. Chlorogenic acid alleviates hyperglycemia-induced cardiac fibrosis through activation of the NO/cGMP/PKG pathway in cardiac fibroblasts. Mol Nutr Food Res. 2021; 65:e2000810.34. Li Q, Zhang Y, Wu N, Yin N, Sun XH, Wang Z. Activation of somatostatin receptor 5 suppresses T-type Ca2+ channels through NO/cGMP/PKG signaling pathway in rat retinal ganglion cells. Neurosci Lett. 2019; 708:134337.35. de Gonzalo-Calvo D, van der Meer RW, Rijzewijk LJ, Smit JW, Revuelta-Lopez E, Nasarre L, et al. Serum microRNA-1 and microRNA-133a levels reflect myocardial steatosis in uncomplicated type 2 diabetes. Sci Rep. 2017; 7:47.36. Nandi SS, Zheng H, Sharma NM, Shahshahan HR, Patel KP, Mishra PK. Lack of miR-133a decreases contractility of diabetic hearts: a role for novel cross talk between tyrosine aminotransferase and tyrosine hydroxylase. Diabetes. 2016; 65:3075–90.37. Gonzalez-Lopez P, Ares-Carral C, Lopez-Pastor AR, InfanteMenendez J, Gonzalez Illaness T, Vega de Ceniga M, et al. Implication of miR-155-5p and miR-143-3p in the vascular insulin resistance and instability of human and experimental atherosclerotic plaque. Int J Mol Sci. 2022; 23:10253.38. Ying W, Gao H, Dos Reis FC, Bandyopadhyay G, Ofrecio JM, Luo Z, et al. MiR-690, an exosomal-derived miRNA from M2-polarized macrophages, improves insulin sensitivity in obese mice. Cell Metab. 2021; 33:781–90.39. Mattisson IY, Bjorkbacka H, Wigren M, Edsfeldt A, Melander O, Fredrikson GN, et al. Elevated markers of death receptoractivated apoptosis are associated with increased risk for development of diabetes and cardiovascular disease. EBioMedicine. 2017; 26:187–97.40. Gong DD, Yu J, Yu JC, Jiang XD. Effect of miR-26a targeting GSK-3β/β-catenin signaling pathway on myocardial apoptosis in rats with myocardial ischemia-reperfusion. Eur Rev Med Pharmacol Sci. 2019; 23:7073–82.41. Tong L, Li W, Zhang Y, Zhou F, Zhao Y, Zhao L, et al. Tacrolimus inhibits insulin release and promotes apoptosis of Min6 cells through the inhibition of the PI3K/Akt/mTOR pathway. Mol Med Rep. 2021; 24:658.42. Forzisi E, Yu W, Rajwade P, Sesti F. Antagonistic roles of RasMAPK and Akt signaling in integrin-K+ channel complexmediated cellular apoptosis. FASEB J. 2022; 36:e22292.43. Adel FW, Zheng Y, Wan SH, Greason C, Pan S, Ameenuddin S, et al. Insulin therapy is associated with increased myocardial interstitial fibrosis and cardiomyocyte apoptosis in a rodent model of experimental diabetes. Front Physiol. 2022; 13:890907.44. Park MJ, Han HJ, Kim DI. Lipotoxicity-induced PRMT1 exacerbates mesangial cell apoptosis via endoplasmic reticulum stress. Int J Mol Sci. 2017; 18:1421.45. Wu L, Guo T, Deng R, Liu L, Yu Y. Apigenin ameliorates insulin resistance and lipid accumulation by endoplasmic reticulum stress and SREBP-1c/SREBP-2 pathway in palmitate-induced HepG2 cells and high-fat diet-fed mice. J Pharmacol Exp Ther. 2021; 377:146–56.46. Dewanjee S, Vallamkondu J, Kalra RS, John A, Reddy PH, Kandimalla R. Autophagy in the diabetic heart: a potential pharmacotherapeutic target in diabetic cardiomyopathy. Ageing Res Rev. 2021; 68:101338.47. Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res. 2018; 122:624–38.48. Frendo-Cumbo S, Tokarz VL, Bilan PJ, Brumell JH, Klip A. Communication between autophagy and insulin action: at the crux of insulin action-insulin resistance? Front Cell Dev Biol. 2021; 9:708431.49. Han K, Jia N, Zhong Y, Shang X. S14G-humanin alleviates insulin resistance and increases autophagy in neurons of APP/PS1 transgenic mouse. J Cell Biochem. 2018; 119:3111–7.50. Zhou W, Ye S. Rapamycin improves insulin resistance and hepatic steatosis in type 2 diabetes rats through activation of autophagy. Cell Biol Int. 2018; 42:1282–91.51. Frendo-Cumbo S, Jaldin-Fincati JR, Coyaud E, Laurent EM, Townsend LK, Tan JM, et al. Deficiency of the autophagy gene ATG16L1 induces insulin resistance through KLHL9/KLHL13/CUL3-mediated IRS1 degradation. J Biol Chem. 2019; 294:16172–85.52. Jaganathan R, Ravindran R, Dhanasekaran S. Emerging role of adipocytokines in type 2 diabetes as mediators of insulin resistance and cardiovascular disease. Can J Diabetes. 2018; 42:446–56.53. Yang F, Xue L, Han Z, Xu F, Cao S, Dai S, et al. Vaspin alleviates myocardial ischaemia/reperfusion injury via activating autophagic flux and restoring lysosomal function. Biochem Biophys Res Commun. 2018; 503:501–7.54. Li X, Ke X, Li Z, Li B. Vaspin prevents myocardial injury in rats model of diabetic cardiomyopathy by enhancing autophagy and inhibiting inflammation. Biochem Biophys Res Commun. 2019; 514:1–8.55. Wang X, Chen X, Zhou W, Men H, Bao T, Sun Y, et al. Ferroptosis is essential for diabetic cardiomyopathy and is prevented by sulforaphane via AMPK/NRF2 pathways. Acta Pharm Sin B. 2022; 12:708–22.56. Li D, Jiang C, Mei G, Zhao Y, Chen L, Liu J, et al. Quercetin alleviates ferroptosis of pancreatic β cells in type 2 diabetes. Nutrients. 2020; 12:2954.57. Schwarzler J, Mayr L, Radlinger B, Grabherr F, Philipp M, Texler B, et al. Adipocyte GPX4 protects against inflammation, hepatic insulin resistance and metabolic dysregulation. Int J Obes (Lond). 2022; 46:951–9.58. Fang X, Cai Z, Wang H, Han D, Cheng Q, Zhang P, et al. Loss of cardiac ferritin H facilitates cardiomyopathy via Slc7a11-mediated ferroptosis. Circ Res. 2020; 127:486–501.59. Wang G, Song X, Zhao L, Li Z, Liu B. Resveratrol prevents diabetic cardiomyopathy by increasing Nrf2 expression and transcriptional activity. Biomed Res Int. 2018; 2018:2150218.60. Cianflone E, Torella M, Biamonte F, De Angelis A, Urbanek K, Costanzo FS, et al. Targeting cardiac stem cell senescence to treat cardiac aging and disease. Cells. 2020; 9:1558.61. Vecellio M, Spallotta F, Nanni S, Colussi C, Cencioni C, Derlet A, et al. The histone acetylase activator pentadecylidenemalonate 1b rescues proliferation and differentiation in the human cardiac mesenchymal cells of type 2 diabetic patients. Diabetes. 2014; 63:2132–47.62. Rouault C, Marcelin G, Adriouch S, Rose C, Genser L, Ambrosini M, et al. Senescence-associated β-galactosidase in subcutaneous adipose tissue associates with altered glycaemic status and truncal fat in severe obesity. Diabetologia. 2021; 64:240–54.63. Roos CM, Zhang B, Palmer AK, Ogrodnik MB, Pirtskhalava T, Thalji NM, et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell. 2016; 15:973–7.64. Westergren HU, Svedlund S, Momo RA, Blomster JI, Wahlander K, Rehnstrom E, et al. Insulin resistance, endothelial function, angiogenic factors and clinical outcome in non-diabetic patients with chest pain without myocardial perfusion defects. Cardiovasc Diabetol. 2016; 15:36.65. Hill MA, Yang Y, Zhang L, Sun Z, Jia G, Parrish AR, et al. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism. 2021; 119:154766.66. Muniyappa R, Chen H, Montagnani M, Sherman A, Quon MJ. Endothelial dysfunction due to selective insulin resistance in vascular endothelium: insights from mechanistic modeling. Am J Physiol Endocrinol Metab. 2020; 319:E629–46.67. Gao H, Wang X, Lin C, An Z, Yu J, Cao H, et al. Exosomal MALAT1 derived from ox-LDL-treated endothelial cells induce neutrophil extracellular traps to aggravate atherosclerosis. Biol Chem. 2020; 401:367–76.68. Zhang H, Liu J, Qu D, Wang L, Wong CM, Lau CW, et al. Serum exosomes mediate delivery of arginase 1 as a novel mechanism for endothelial dysfunction in diabetes. Proc Natl Acad Sci U S A. 2018; 115:E6927–36.69. Wang S, Zhan J, Lin X, Wang Y, Wang Y, Liu Y. CircRNA-0077930 from hyperglycaemia-stimulated vascular endothelial cell exosomes regulates senescence in vascular smooth muscle cells. Cell Biochem Funct. 2020; 38:1056–68.70. Jain R, Awal H, Sen S. Using adult stem cells to monitor endothelial dysfunction in diabetes mellitus. J Diabetes Complications. 2020; 34:107588.71. Peyter AC, Armengaud JB, Guillot E, Yzydorczyk C. Endothelial progenitor cells dysfunctions and cardiometabolic disorders: from mechanisms to therapeutic approaches. Int J Mol Sci. 2021; 22:6667.72. Fadini GP, de Kreutzenberg SV, Coracina A, Baesso I, Agostini C, Tiengo A, et al. Circulating CD34+ cells, metabolic syndrome, and cardiovascular risk. Eur Heart J. 2006; 27:2247–55.73. Haberzettl P, McCracken JP, Bhatnagar A, Conklin DJ. Insulin sensitizers prevent fine particulate matter-induced vascular insulin resistance and changes in endothelial progenitor cell homeostasis. Am J Physiol Heart Circ Physiol. 2016; 310:H1423–38.74. Dai X, Wang K, Fan J, Liu H, Fan X, Lin Q, et al. Nrf2 transcriptional upregulation of IDH2 to tune mitochondrial dynamics and rescue angiogenic function of diabetic EPCs. Redox Biol. 2022; 56:102449.75. Hinkel R, Howe A, Renner S, Ng J, Lee S, Klett K, et al. Diabetes mellitus-induced microvascular destabilization in the myocardium. J Am Coll Cardiol. 2017; 69:131–43.76. Shikama M, Sonoda N, Morimoto A, Suga S, Tajima T, Kozawa J, et al. Association of crossing capillaries in the finger nailfold with diabetic retinopathy in type 2 diabetes mellitus. J Diabetes Investig. 2021; 12:1007–14.77. O’Brien KD, McDonald TO, Chait A, Allen MD, Alpers CE. Neovascular expression of E-selectin, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 in human atherosclerosis and their relation to intimal leukocyte content. Circulation. 1996; 93:672–82.78. Owusu J, Barrett E. Early microvascular dysfunction: is the vasa vasorum a “missing link” in insulin resistance and atherosclerosis. Int J Mol Sci. 2021; 22:7574.79. Wang F, Chen FF, Shang YY, Li Y, Wang ZH, Han L, et al. Insulin resistance adipocyte-derived exosomes aggravate atherosclerosis by increasing vasa vasorum angiogenesis in diabetic ApoE-/- mice. Int J Cardiol. 2018; 265:181–7.80. Tanida M, Imanishi K, Akashi H, Kurata Y, Chonan O, Naito E, et al. Injection of Lactobacillus casei strain Shirota affects autonomic nerve activities in a tissue-specific manner, and regulates glucose and lipid metabolism in rats. J Diabetes Investig. 2014; 5:153–61.81. Lundqvist MH, Almby K, Wiklund U, Abrahamsson N, Kamble PG, Pereira MJ, et al. Altered hormonal and autonomic nerve responses to hypo- and hyperglycaemia are found in overweight and insulin-resistant individuals and may contribute to the development of type 2 diabetes. Diabetologia. 2021; 64:641–55.82. Sposato V, Canu N, Fico E, Fusco S, Bolasco G, Ciotti MT, et al. The medial septum is insulin resistant in the AD presymptomatic phase: rescue by nerve growth factor-driven IRS1 activation. Mol Neurobiol. 2019; 56:535–52.83. Oza MJ, Kulkarni YA. Formononetin ameliorates diabetic neuropathy by increasing expression of SIRT1 and NGF. Chem Biodivers. 2020; 17:e2000162.84. Wang HJ, Li YL, Gao L, Zucker IH, Wang W. Alteration in skeletal muscle afferents in rats with chronic heart failure. J Physiol. 2010; 588(Pt 24s):5033–47.85. Xu X, Liu B, Yang J, Zou Y, Sun M, Li Z, et al. Glucokinase in stellate ganglia cooperates with P2X3 receptor to develop cardiac sympathetic neuropathy in type 2 diabetes rats. Brain Res Bull. 2020; 165:290–7.86. Limberg JK, Soares RN, Padilla J. Role of the autonomic nervous system in the hemodynamic response to hyperinsulinemia-implications for obesity and insulin resistance. Curr Diab Rep. 2022; 22:169–75.87. Yu Y, Wei SG, Zhang ZH, Weiss RM, Felder RB. ERK1/2 MAPK signaling in hypothalamic paraventricular nucleus contributes to sympathetic excitation in rats with heart failure after myocardial infarction. Am J Physiol Heart Circ Physiol. 2016; 310:H732–9.88. Bozkurt B, Mann DL, Deswal A. Biomarkers of inflammation in heart failure. Heart Fail Rev. 2010; 15:331–41.89. Festa A, D’Agostino R Jr, Howard G, Mykkanen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS). Circulation. 2000; 102:42–7.90. Fuentes-Antras J, Ioan AM, Tunon J, Egido J, Lorenzo O. Activation of toll-like receptors and inflammasome complexes in the diabetic cardiomyopathy-associated inflammation. Int J Endocrinol. 2014; 2014:847827.91. Orliaguet L, Ejlalmanesh T, Alzaid F. Metabolic and molecular mechanisms of macrophage polarisation and adipose tissue insulin resistance. Int J Mol Sci. 2020; 21:5731.92. Luo B, Li B, Wang W, Liu X, Xia Y, Zhang C, et al. NLRP3 gene silencing ameliorates diabetic cardiomyopathy in a type 2 diabetes rat model. PLoS One. 2014; 9:e104771.93. Menegazzo L, Ciciliot S, Poncina N, Mazzucato M, Persano M, Bonora B, et al. NETosis is induced by high glucose and associated with type 2 diabetes. Acta Diabetol. 2015; 52:497–503.94. Wang Y, Sano S, Oshima K, Sano M, Watanabe Y, Katanasaka Y, et al. Wnt5a-mediated neutrophil recruitment has an obligatory role in pressure overload-induced cardiac dysfunction. Circulation. 2019; 140:487–99.95. Nahrendorf M, Swirski FK. Immunology: neutrophil-macrophage communication in inflammation and atherosclerosis. Science. 2015; 349:237–8.96. Njeim R, Azar WS, Fares AH, Azar ST, Kfoury Kassouf H, Eid AA. NETosis contributes to the pathogenesis of diabetes and its complications. J Mol Endocrinol. 2020; 65:R65–76.97. Wilson AJ, Gill EK, Abudalo RA, Edgar KS, Watson CJ, Grieve DJ. Reactive oxygen species signalling in the diabetic heart: emerging prospect for therapeutic targeting. Heart. 2018; 104:293–9.98. Gray SP, Di Marco E, Okabe J, Szyndralewiez C, Heitz F, Montezano AC, et al. NADPH oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation. 2013; 127:1888–902.99. Kinsara AJ, Ismail YM. Metformin in heart failure patients. Indian Heart J. 2018; 70:175–6.100. Sardu C, Paolisso P, Sacra C, Mauro C, Minicucci F, Portoghese M, et al. Effects of metformin therapy on coronary endothelial dysfunction in patients with prediabetes with stable angina and nonobstructive coronary artery stenosis: the CODYCE Multicenter Prospective Study. Diabetes Care. 2019; 42:1946–55.101. Top WM, Kooy A, Stehouwer CD. Metformin: a narrative review of its potential benefits for cardiovascular disease, cancer and dementia. Pharmaceuticals (Basel). 2022; 15:312.102. Ladeiras-Lopes R, Sampaio F, Leite S, Santos-Ferreira D, Vilela E, Leite-Moreira A, et al. Metformin in non-diabetic patients with metabolic syndrome and diastolic dysfunction: the METDIME randomized trial. Endocrine. 2021; 72:699–710.103. Dandona P, Ghanim H, Chaudhuri A, Mohanty P. Thiazolidinediones-improving endothelial function and potential long-term benefits on cardiovascular disease in subjects with type 2 diabetes. J Diabetes Complications. 2008; 22:62–75.104. de Jong M, van der Worp HB, van der Graaf Y, Visseren FL, Westerink J. Pioglitazone and the secondary prevention of cardiovascular disease: a meta-analysis of randomized-controlled trials. Cardiovasc Diabetol. 2017; 16:134.105. Tian Y, Chen T, Wu Y, Yang L, Wang L, Fan X, et al. Pioglitazone stabilizes atherosclerotic plaque by regulating the Th17/Treg balance in AMPK-dependent mechanisms. Cardiovasc Diabetol. 2017; 16:140.106. Liakos A, Lambadiari V, Bargiota A, Kitsios K, Avramidis I, Kotsa K, et al. Effect of liraglutide on ambulatory blood pressure in patients with hypertension and type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2019; 21:517–24.107. Koseoglu D, Koparal SS, Ozdemir Baser O, Berker D. Exenatide improves cardiovascular risk factors in obese patients with type 2 diabetes mellitus: a prospective study. Turk J Med Sci. 2021; 51:167–74.108. Zheng W, Zhou J, Song S, Kong W, Xia W, Chen L, et al. Dipeptidyl-peptidase 4 inhibitor sitagliptin ameliorates hepatic insulin resistance by modulating inflammation and autophagy in ob/ob Mice. Int J Endocrinol. 2018; 2018:8309723.109. Zannad F, Cannon CP, Cushman WC, Bakris GL, Menon V, Perez AT, et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015; 385:2067–76.110. Wang X, Ni J, Guo R, Li L, Su J, He F, et al. SGLT2 inhibitors break the vicious circle between heart failure and insulin resistance: targeting energy metabolism. Heart Fail Rev. 2022; 27:961–80.111. Merovci A, Solis-Herrera C, Daniele G, Eldor R, Fiorentino TV, Tripathy D, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest. 2014; 124:509–14.112. Kashiwagi A, Maegawa H. Metabolic and hemodynamic effects of sodium-dependent glucose cotransporter 2 inhibitors on cardio-renal protection in the treatment of patients with type 2 diabetes mellitus. J Diabetes Investig. 2017; 8:416–27.113. Bonaventura A, Carbone S, Dixon DL, Abbate A, Montecucco F. Pharmacologic strategies to reduce cardiovascular disease in type 2 diabetes mellitus: focus on SGLT-2 inhibitors and GLP-1 receptor agonists. J Intern Med. 2019; 286:16–31.114. Koh KK, Sakuma I, Quon MJ. Differential metabolic effects of distinct statins. Atherosclerosis. 2011; 215:1–8.115. Yandrapalli S, Malik A, Guber K, Rochlani Y, Pemmasani G, Jasti M, et al. Statins and the potential for higher diabetes mellitus risk. Expert Rev Clin Pharmacol. 2019; 12:825–30.116. Duhaney TA, Cui L, Rude MK, Lebrasseur NK, Ngoy S, De Silva DS, et al. Peroxisome proliferator-activated receptor alpha-independent actions of fenofibrate exacerbates left ventricular dilation and fibrosis in chronic pressure overload. Hypertension. 2007; 49:1084–94.117. Bhatt DL, Eikelboom JW, Connolly SJ, Steg PG, Anand SS, Verma S, et al. Role of combination antiplatelet and anticoagulation therapy in diabetes mellitus and cardiovascular disease: insights from the COMPASS Trial. Circulation. 2020; 141:1841–54.118. Brown E, Ozawa K, Moccetti F, Vinson A, Hodovan J, Nguyen TA, et al. Arterial platelet adhesion in atherosclerosis-prone arteries of obese, insulin-resistant nonhuman primates. J Am Heart Assoc. 2021; 10:e019413.119. Liu M, Zhuang X, Chen X, Zhang S, Yang D, Zhong X, et al. Antiplatelet strategy in primary and secondary prevention of cardiovascular disease in patients with type 2 diabetes mellitus: a perspective from the guideline appraisal. J Diabetes Investig. 2021; 12:99–108.120. Nemati M, Karbalaei N, Mokarram P, Dehghani F. Effects of platelet-rich plasma on the pancreatic islet survival and function, islet transplantation outcome and pancreatic pdx1 and insulin gene expression in streptozotocin-induced diabetic rats. Growth Factors. 2020; 38:137–51.121. Zhang W, Dun Y, You B, Qiu L, Ripley-Gonzalez JW, Cheng J, et al. Trimetazidine and exercise offer analogous improvements to the skeletal muscle insulin resistance of mice through Nrf2 signaling. BMJ Open Diabetes Res Care. 2022; 10:e002699.122. Murarka S, Movahed MR. Diabetic cardiomyopathy. J Card Fail. 2010; 16:971–9.123. Ruggenenti P, Cravedi P, Remuzzi G. The RAAS in the pathogenesis and treatment of diabetic nephropathy. Nat Rev Nephrol. 2010; 6:319–30.124. Kristensen SL, Preiss D, Jhund PS, Squire I, Cardoso JS, Merkely B, et al. Risk related to pre-diabetes mellitus and diabetes mellitus in heart failure with reduced ejection fraction: insights from prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial. Circ Heart Fail. 2016; 9:e002560.125. Wu W, Shi F, Liu D, Ceddia RP, Gaffin R, Wei W, et al. Enhancing natriuretic peptide signaling in adipose tissue, but not in muscle, protects against diet-induced obesity and insulin resistance. Sci Signal. 2017; 10:eaam6870.126. Gonzalez-Juanatey JR, Gorriz JL, Ortiz A, Valle A, Soler MJ, Facila L. Cardiorenal benefits of finerenone: protecting kidney and heart. Ann Med. 2023; 55:502–13.127. Pitt B, Filippatos G, Agarwal R, Anker SD, Bakris GL, Rossing P, et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med. 2021; 385:2252–63.128. Filippatos G, Anker SD, Agarwal R, Ruilope LM, Rossing P, Bakris GL, et al. Finerenone reduces risk of incident heart failure in patients with chronic kidney disease and type 2 diabetes: analyses from the FIGARO-DKD Trial. Circulation. 2022; 145:437–47.129. Ramsay LE, Yeo WW, Jackson PR. Influence of diuretics, calcium antagonists, and alpha-blockers on insulin sensitivity and glucose tolerance in hypertensive patients. J Cardiovasc Pharmacol. 1992; 20 Suppl 11:S49–54.130. Reid JL. The place of alpha blockers in the treatment of hypertension. Clin Exp Hypertens. 1993; 15:1291–7.131. Haas SJ, Vos T, Gilbert RE, Krum H. Are beta-blockers as efficacious in patients with diabetes mellitus as in patients without diabetes mellitus who have chronic heart failure?: a meta-analysis of large-scale clinical trials. Am Heart J. 2003; 146:848–53.132. Wang X, Feng Z, Wang X, Yang L, Han S, Cao K, et al. O-GlcNA case deficiency suppresses skeletal myogenesis and insulin sensitivity in mice through the modulation of mitochondrial homeostasis. Diabetologia. 2016; 59:1287–96.133. Hu Y, Belke D, Suarez J, Swanson E, Clark R, Hoshijima M, et al. Adenovirus-mediated overexpression of O-GlcNAcase improves contractile function in the diabetic heart. Circ Res. 2005; 96:1006–13.134. Campbell H, Aguilar-Sanchez Y, Quick AP, Dobrev D, Wehrens XH. SPEG: a key regulator of cardiac calcium homeostasis. Cardiovasc Res. 2021; 117:2175–85.135. Chen Q, Chen X, Han C, Wang Y, Huang T, Du Y, et al. FGF-2 transcriptionally down-regulates the expression of BNIP3L via PI3K/Akt/FoxO3a signaling and inhibits necrosis and mitochondrial dysfunction induced by high concentrations of hydrogen peroxide in H9c2 cells. Cell Physiol Biochem. 2016; 40:1678–91.136. Ge Q, Xie XX, Xiao X, Li X. Exosome-like vesicles as new mediators and therapeutic targets for treating insulin resistance and β-cell mass failure in type 2 diabetes mellitus. J Diabetes Res. 2019; 2019:3256060.137. Kranendonk ME, Visseren FL, van Balkom BW, Nolte-‘t Hoen EN, van Herwaarden JA, de Jager W, et al. Human adipocyte extracellular vesicles in reciprocal signaling between adipocytes and macrophages. Obesity (Silver Spring). 2014; 22:1296–308.138. James DE, Stockli J, Birnbaum MJ. The aetiology and molecular landscape of insulin resistance. Nat Rev Mol Cell Biol. 2021; 22:751–71.139. Hayden MR. The mighty mitochondria are unifying organelles and metabolic hubs in multiple organs of obesity, insulin resistance, metabolic syndrome, and type 2 diabetes: an observational ultrastructure study. Int J Mol Sci. 2022; 23:4820.140. Kwon B, Gamache T, Lee HK, Querfurth HW. Synergistic effects of β-amyloid and ceramide-induced insulin resistance on mitochondrial metabolism in neuronal cells. Biochim Biophys Acta. 2015; 1852:1810–23.141. Wang H, Chen X, Chen C, Pan T, Li M, Yao L, et al. Electroacupuncture at lower he-sea and front-mu acupoints ameliorates insulin resistance in type 2 diabetes mellitus by regulating the intestinal flora and gut barrier. Diabetes Metab Syndr Obes. 2022; 15:2265–76.142. Hsieh FC, Lee CL, Chai CY, Chen WT, Lu YC, Wu CS. Oral administration of Lactobacillus reuteri GMNL-263 improves insulin resistance and ameliorates hepatic steatosis in high fructose-fed rats. Nutr Metab (Lond). 2013; 10:35.143. Kim D, Seo J, Ha KH, Kim DJ. Maintaining physical activity is associated with reduced major adverse cardiovascular events in people newly diagnosed with diabetes. J Obes Metab Syndr. 2022; 31:187–95.144. Li M, Chi X, Wang Y, Setrerrahmane S, Xie W, Xu H. Trends in insulin resistance: insights into mechanisms and therapeutic strategy. Signal Transduct Target Ther. 2022; 7:216.145. Andreadi A, Bellia A, Di Daniele N, Meloni M, Lauro R, Della-Morte D, et al. The molecular link between oxidative stress, insulin resistance, and type 2 diabetes: a target for new therapies against cardiovascular diseases. Curr Opin Pharmacol. 2022; 62:85–96.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Using Motivational Interviewing to Overcome Psychological Insulin Resistance
- Glut4 in the insulin resistance of NIDDM
- Psychological Insulin Resistance: Key Factors and Intervention
- Insulin Resistance and Insulin Resistance Syndrome
- Letter: Adipokines and Insulin Resistance According to Characteristics of Pregnant Women with Gestational Diabetes Mellitus (Diabetes Metab J 2017;41:457-65)