Clin Endosc.
2024 Jan;57(1):112-121. 10.5946/ce.2022.278.
Impact of sarcopenia on biliary drainage during neoadjuvant therapy for pancreatic cancer
- Affiliations
-
- 1Department of Gastroenterology and Hepatology, Nagoya University Graduate School of Medicine, Nagoya, Japan
- 2Department of Gastroenterological Surgery, Nagoya University Graduate School of Medicine, Nagoya, Japan
- 3Department of Clinical Oncology and Chemotherapy, Nagoya University Graduate School of Medicine, Nagoya, Japan
- 4Department of Surgical Oncology, Nagoya University Graduate School of Medicine, Nagoya, Japan
- KMID: 2551200
- DOI: http://doi.org/10.5946/ce.2022.278
Abstract
- Background/Aims
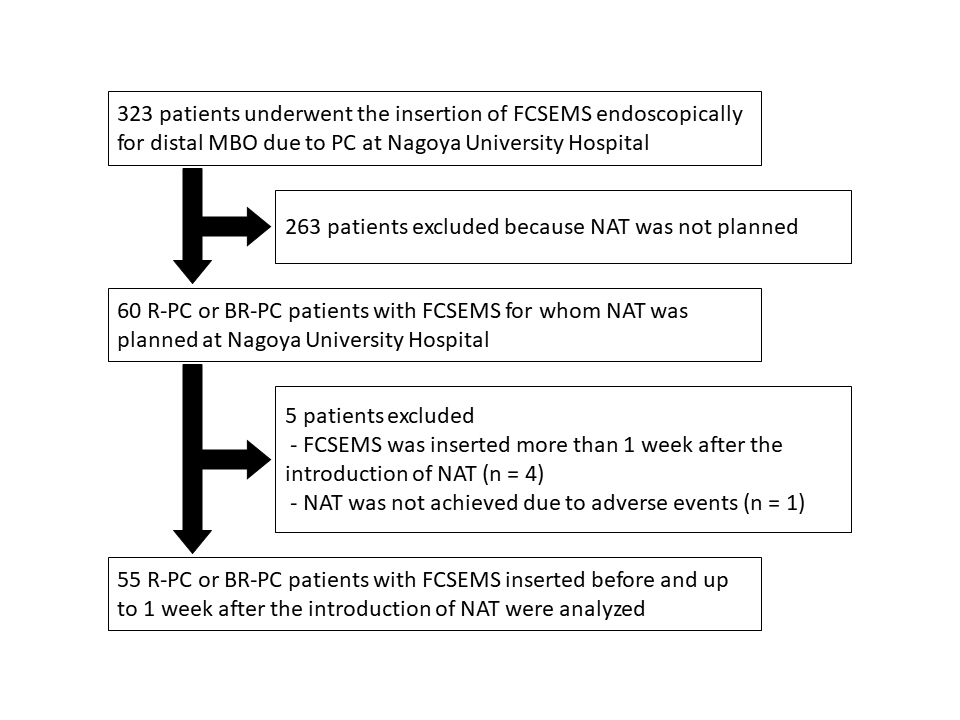
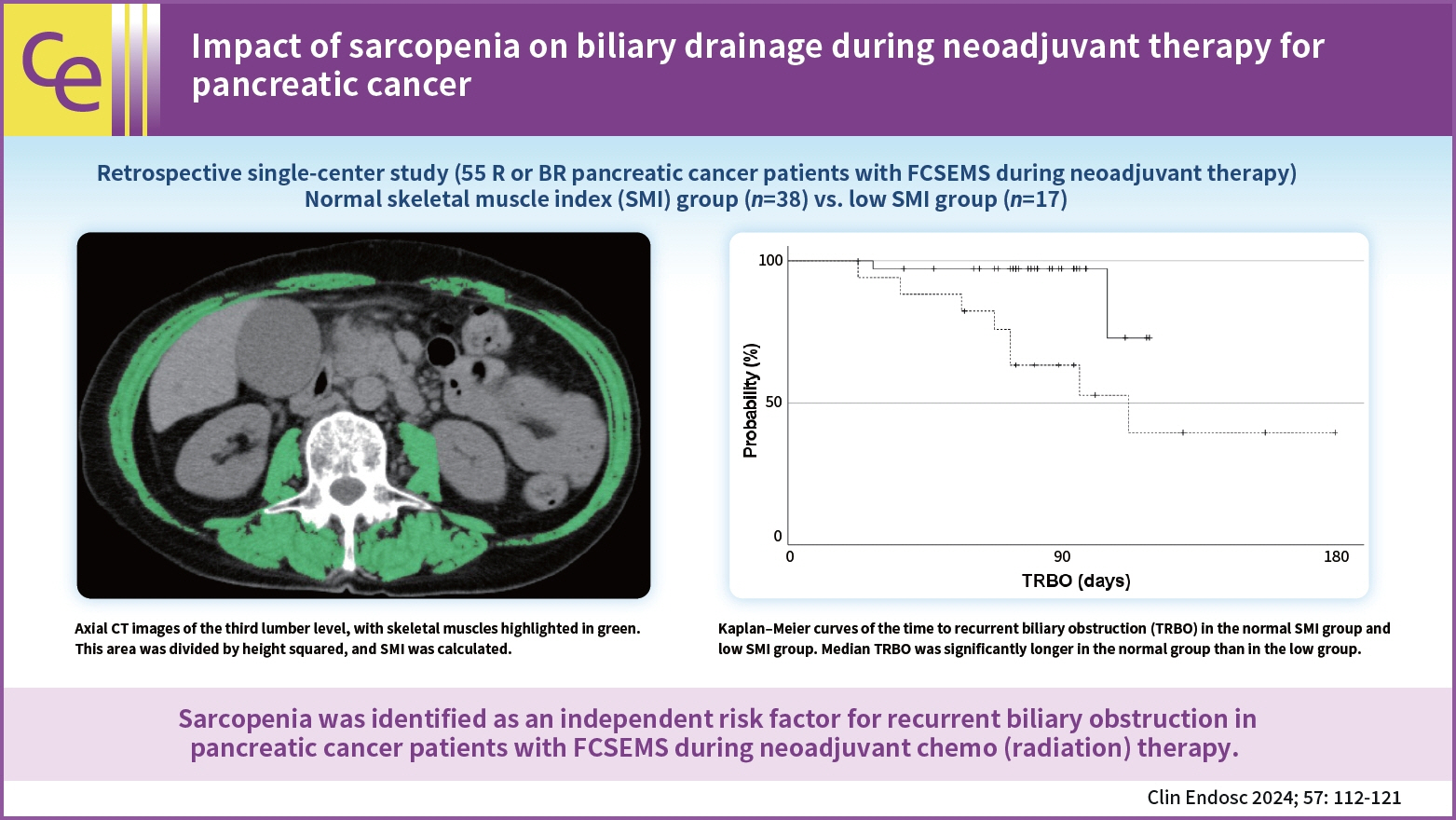
Since the usefulness of neoadjuvant chemo(radiation) therapy (NAT) for pancreatic cancer has been demonstrated, recurrent biliary obstruction (RBO) in patients with pancreatic cancer with a fully covered self-expandable metal stent (FCSEMS) during NAT is expected to increase. This study investigated the impact of sarcopenia on RBO in this setting.
Methods
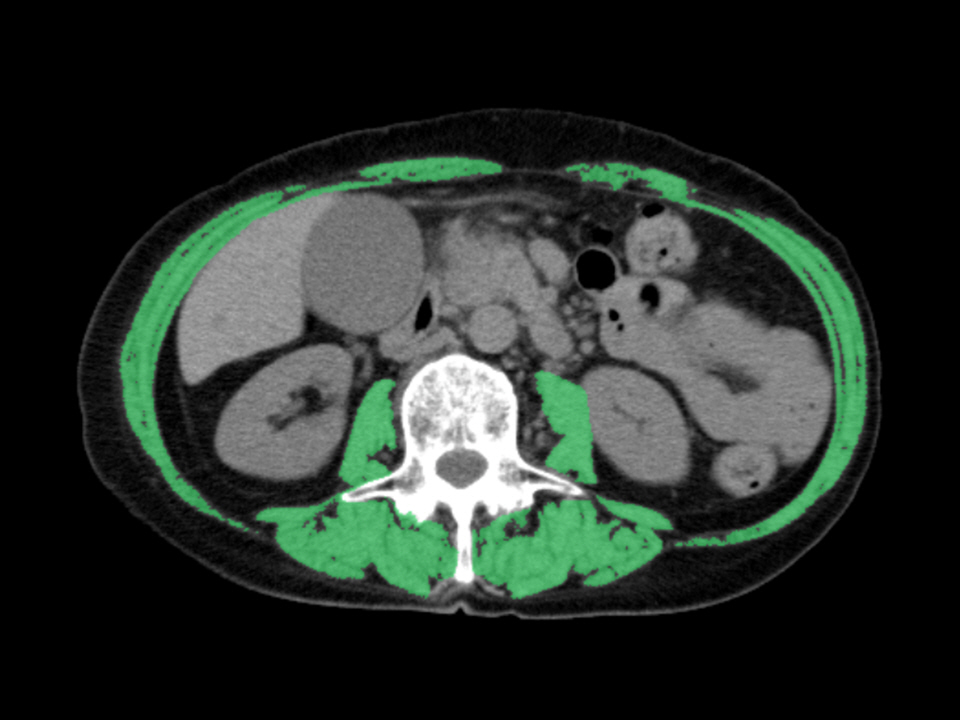
Patients were divided into normal and low skeletal muscle index (SMI) groups and retrospectively analyzed. Patient characteristics, overall survival, time to RBO (TRBO), stent-related adverse events, and postoperative complications were compared between the two groups. A Cox proportional hazard model was used to identify the risk factors for short TRBO.
Results
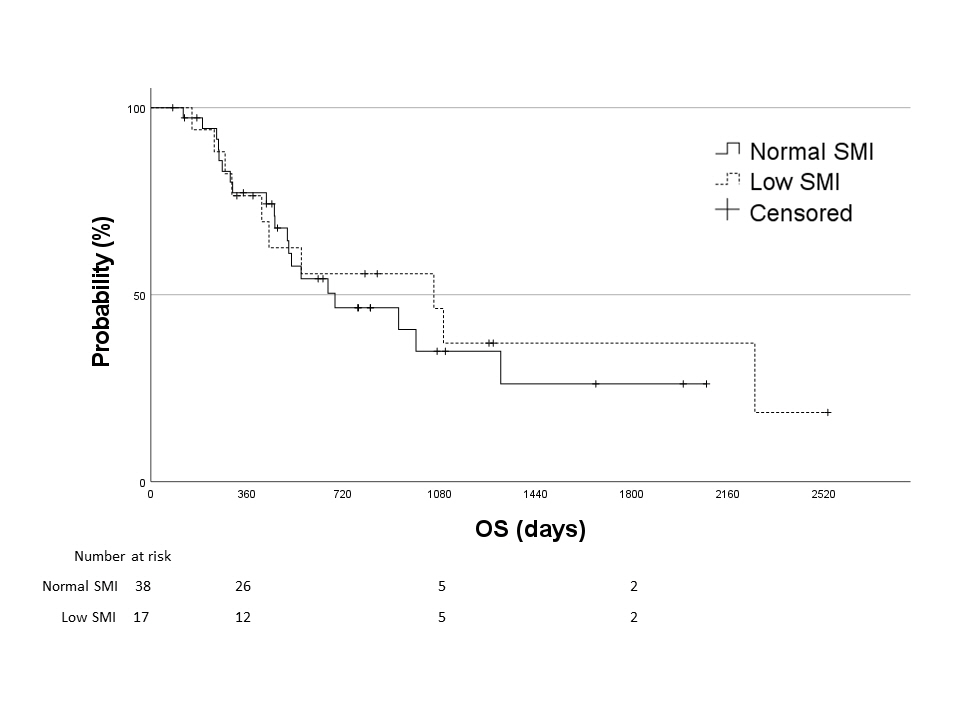
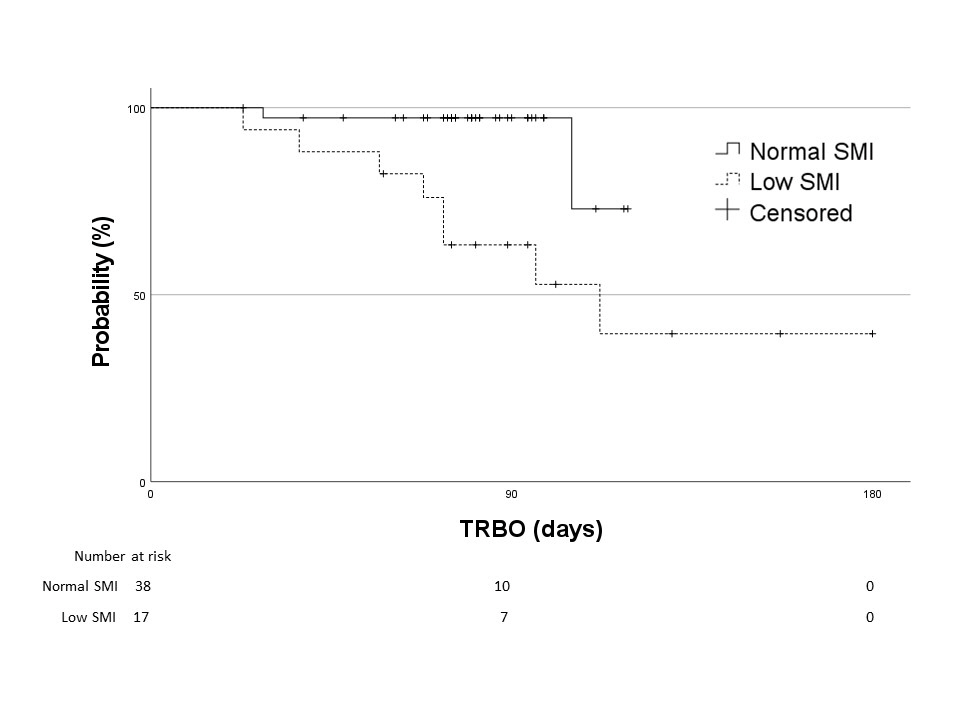
A few significant differences were observed in patient characteristics, overall survival, stent-related adverse events, and postoperative complications between 38 patients in the normal SMI group and 17 in the low SMI group. The median TRBO was not reached in the normal SMI group and was 112 days in the low SMI group (p=0.004). In multivariate analysis, low SMI was the only risk factor for short TRBO, with a hazard ratio of 5.707 (95% confidence interval, 1.148–28.381; p=0.033).
Conclusions
Sarcopenia was identified as an independent risk factor for RBO in patients with pancreatic cancer with FCSEMS during NAT.
Keyword
Figure
Reference
-
1. Motoi F, Kosuge T, Ueno H, et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05). Jpn J Clin Oncol. 2019; 49:190–194.2. Unno M, Hata T, Motoi F. Long-term outcome following neoadjuvant therapy for resectable and borderline resectable pancreatic cancer compared to upfront surgery: a meta-analysis of comparative studies by intention-to-treat analysis. Surg Today. 2019; 49:295–299.3. Yamaguchi J, Yokoyama Y, Fujii T, et al. Results of a phase II study on the use of neoadjuvant chemotherapy (FOLFIRINOX or GEM/nab-PTX) for borderline-resectable pancreatic cancer (NUPAT-01). Ann Surg. 2022; 275:1043–1049.4. Aadam AA, Evans DB, Khan A, et al. Efficacy and safety of self-expandable metal stents for biliary decompression in patients receiving neoadjuvant therapy for pancreatic cancer: a prospective study. Gastrointest Endosc. 2012; 76:67–75.5. Kubota K, Sato T, Watanabe S, et al. Covered self-expandable metal stent deployment promises safe neoadjuvant chemoradiation therapy in patients with borderline resectable pancreatic head cancer. Dig Endosc. 2014; 26:77–86.6. Gardner TB, Spangler CC, Byanova KL, et al. Cost-effectiveness and clinical efficacy of biliary stents in patients undergoing neoadjuvant therapy for pancreatic adenocarcinoma in a randomized controlled trial. Gastrointest Endosc. 2016; 84:460–466.7. Nakamura K, Sho M, Akahori T, et al. A comparison between plastic and metallic biliary stent placement in patients receiving preoperative neoadjuvant chemoradiotherapy for resectable pancreatic cancer. World J Surg. 2019; 43:642–648.8. Kuwatani M, Nakamura T, Hayashi T, et al. Clinical outcomes of biliary drainage during a neoadjuvant therapy for pancreatic cancer: metal versus plastic stents. Gut Liver. 2020; 14:269–273.9. Hasegawa S, Kubota K, Yagi S, et al. Covered metallic stent placement for biliary drainage could be promising in the coming era of neoadjuvant chemo-radiation therapy for all pancreatic cancer. J Hepatobiliary Pancreat Sci. 2021; 28:617–624.10. Hamada T, Isayama H, Nakai Y, et al. Duodenal invasion is a risk factor for the early dysfunction of biliary metal stents in unresectable pancreatic cancer. Gastrointest Endosc. 2011; 74:548–555.11. van Boeckel PG, Steyerberg EW, Vleggaar FP, et al. Multicenter study evaluating factors for stent patency in patients with malignant biliary strictures: development of a simple score model. J Gastroenterol. 2011; 46:1104–1110.12. Nakai Y, Isayama H, Mukai T, et al. Impact of anticancer treatment on recurrent obstruction in covered metallic stents for malignant biliary obstruction. J Gastroenterol. 2013; 48:1293–1299.13. Koya Y, Shibata M, Oe S, et al. Impact of sarcopenia on recurrent biliary obstruction after insertion of self-expandable metallic stent in patients with malignant biliary obstruction. J Hepatobiliary Pancreat Sci. 2021; 28:572–580.14. Nishikawa H, Shiraki M, Hiramatsu A, et al. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol Res. 2016; 46:951–963.15. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004; 240:205–213.16. Isayama H, Hamada T, Yasuda I, et al. TOKYO criteria 2014 for transpapillary biliary stenting. Dig Endosc. 2015; 27:259–264.17. Seo DW, Sherman S, Dua KS, et al. Covered and uncovered biliary metal stents provide similar relief of biliary obstruction during neoadjuvant therapy in pancreatic cancer: a randomized trial. Gastrointest Endosc. 2019; 90:602–612.18. Saito K, Nakai Y, Isayama H, et al. A prospective multicenter study of partially covered metal stents in patients receiving neoadjuvant chemotherapy for resectable and borderline resectable pancreatic cancer: BTS-NAC Study. Gut Liver. 2021; 15:135–141.19. Dumonceau JM, Tringali A, Papanikolaou IS, et al. Endoscopic biliary stenting: indications, choice of stents, and results: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline: updated October 2017. Endoscopy. 2018; 50:910–930.20. Kawashima H, Hashimoto S, Ohno E, et al. Comparison of 8- and 10-mm diameter fully covered self-expandable metal stents: a multicenter prospective study in patients with distal malignant biliary obstruction. Dig Endosc. 2019; 31:439–447.21. Cruz-Jentoft AJ, Landi F, Schneider SM, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review: report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing. 2014; 43:748–759.22. Chen LK, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020; 21:300–307.23. Ohara M, Suda G, Kimura M, et al. Analysis of the optimal psoas muscle mass index cut-off values, as measured by computed tomography, for the diagnosis of loss of skeletal muscle mass in Japanese people. Hepatol Res. 2020; 50:715–725.24. Okumura S, Kaido T, Hamaguchi Y, et al. Impact of preoperative quality as well as quantity of skeletal muscle on survival after resection of pancreatic cancer. Surgery. 2015; 157:1088–1098.25. Okumura S, Kaido T, Hamaguchi Y, et al. Impact of the preoperative quantity and quality of skeletal muscle on outcomes after resection of extrahepatic biliary malignancies. Surgery. 2016; 159:821–833.26. Shintakuya R, Uemura K, Murakami Y, et al. Sarcopenia is closely associated with pancreatic exocrine insufficiency in patients with pancreatic disease. Pancreatology. 2017; 17:70–75.27. Iglesia D, Avci B, Kiriukova M, et al. Pancreatic exocrine insufficiency and pancreatic enzyme replacement therapy in patients with advanced pancreatic cancer: a systematic review and meta-analysis. United European Gastroenterol J. 2020; 8:1115–1125.28. Domínguez-Muñoz JE, de la Iglesia-García D, Nieto-García L, et al. Endoscopic pancreatic drainage improves exocrine pancreatic function in patients with unresectable pancreatic cancer: a double-blind, prospective, randomized, single-center, interventional study. Pancreas. 2021; 50:679–684.29. Leitzmann MF, Giovannucci EL, Rimm EB, et al. The relation of physical activity to risk for symptomatic gallstone disease in men. Ann Intern Med. 1998; 128:417–425.30. Serra-Prat M, Mans E, Palomera E, et al. Gastrointestinal peptides, gastrointestinal motility, and anorexia of aging in frail elderly persons. Neurogastroenterol Motil. 2013; 25:291.31. Nishikawa H, Shiraki M, Hiramatsu A, et al. Reduced handgrip strength predicts poorer survival in chronic liver diseases: a large multicenter study in Japan. Hepatol Res. 2021; 51:957–967.32. Bundred JR, Kamarajah SK, Hammond JS, et al. Prehabilitation prior to surgery for pancreatic cancer: a systematic review. Pancreatology. 2020; 20:1243–1250.33. Nakajima H, Yokoyama Y, Inoue T, et al. Clinical benefit of preoperative exercise and nutritional therapy for patients undergoing hepato-pancreato-biliary surgeries for malignancy. Ann Surg Oncol. 2019; 26:264–272.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Relief of Obstruction in the Management of Pancreatic Cancer
- Preoperative biliary drainage for pancreatic cancer
- Neoadjuvant Therapy for Resectable or Borderline Resectable Pancreatic Cancer
- Updates of Chemotherapy and Radiotherapy for Pancreatic Cancer
- Role of Neoadjuvant Therapy for Borderline Resectable or Locally Advanced Pancreatic Cancer