Acute Crit Care.
2023 Nov;38(4):389-398. 10.4266/acc.2023.01368.
Plasma biomarkers for brain injury in extracorporeal membrane oxygenation
- Affiliations
-
- 1Division of Neurosciences Critical Care and Cardiac Surgery, Departments of Neurology, Surgery, Anesthesiology and Critical Care Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, USA
- 2Division of Neurocritical Care, Department of Neurosurgery, McGovern School of Medicine, University of Texas Health Science Center, Houston, TX, USA
- 3Griffith University School of Medicine, Queensland, Australia
- 4Critical Care Research Group, The Prince Charles Hospital, Queensland, Australia
- KMID: 2550895
- DOI: http://doi.org/10.4266/acc.2023.01368
Abstract
- Extracorporeal membrane oxygenation (ECMO) is a life-saving intervention for patients with refractory cardiorespiratory failure. Despite its benefits, ECMO carries a significant risk of neurological complications, including acute brain injury (ABI). Although standardized neuromonitoring and neurological care have been shown to improve early detection of ABI, the inability to perform neuroimaging in a timely manner is a major limitation in the accurate diagnosis of neurological complications. Therefore, blood-based biomarkers capable of detecting ongoing brain injury at the bedside are of great clinical significance. This review aims to provide a concise review of the current literature on plasma biomarkers for ABI in patients on ECMO support.
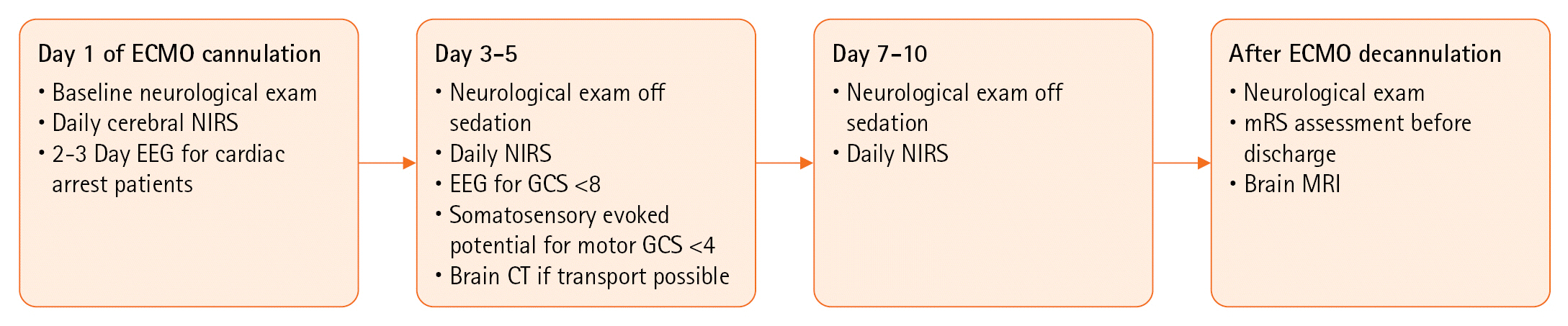
Figure
Reference
-
1. Lang NW, Schwihla I, Weihs V, Kasparek M, Joestl J, Hajdu S, et al. Survival rate and outcome of extracorporeal life support (ECLS) for treatment of acute cardiorespiratory failure in trauma patients. Sci Rep. 2019; 9:12902.
Article2. Migdady I, Rice C, Deshpande A, Hernandez AV, Price C, Whitman GJ, et al. Brain injury and neurologic outcome in patients undergoing extracorporeal cardiopulmonary resuscitation: a systematic review and meta-analysis. Crit Care Med. 2020; 48:e611–9.
Article3. Cho SM, Ziai W, Mayasi Y, Gusdon AM, Creed J, Sharrock M, et al. Noninvasive neurological monitoring in extracorporeal membrane oxygenation. ASAIO J. 2020; 66:388–93.
Article4. Ong CS, Etchill E, Dong J, Shou BL, Shelley L, Giuliano K, et al. Neuromonitoring detects brain injury in patients receiving extracorporeal membrane oxygenation support. J Thorac Cardiovasc Surg. 2023; 165:2104–10.
Article5. Cho SM, Wilcox C, Keller S, Acton M, Rando H, Etchill E, et al. Assessing the SAfety and FEasibility of bedside portable low-field brain Magnetic Resonance Imaging in patients on ECMO (SAFE-MRI ECMO study): study protocol and first case series experience. Crit Care. 2022; 26:119.
Article6. Kim YJ, Kim YH, Youn CS, Cho IS, Kim SJ, Wee JH, et al. Different neuroprognostication thresholds of neuron-specific enolase in shockable and non-shockable out-of-hospital cardiac arrest: a prospective multicenter observational study in Korea (the KORHN-PRO registry). Crit Care. 2023; 27:313.
Article7. Gul SS, Huesgen KW, Wang KK, Mark K, Tyndall JA. Prognostic utility of neuroinjury biomarkers in post out-of-hospital cardiac arrest (OHCA) patient management. Med Hypotheses. 2017; 105:34–47.
Article8. Lee D, Cho Y, Ko Y, Heo NH, Kang HG, Han S. Neuron-specific enolase level as a predictor of neurological outcome in near-hanging patients: a retrospective multicenter study. PLoS One. 2021; 16:e0246898.
Article9. Bembea MM, Rizkalla N, Freedy J, Barasch N, Vaidya D, Pronovost PJ, et al. Plasma biomarkers of brain injury as diagnostic tools and outcome predictors after extracorporeal membrane oxygenation. Crit Care Med. 2015; 43:2202–11.
Article10. Floerchinger B, Philipp A, Camboni D, Foltan M, Lunz D, Lubnow M, et al. NSE serum levels in extracorporeal life support patients: relevance for neurological outcome? Resuscitation. 2017; 121:166–71.
Article11. Ramont L, Thoannes H, Volondat A, Chastang F, Millet MC, Maquart FX. Effects of hemolysis and storage condition on neuron-specific enolase (NSE) in cerebrospinal fluid and serum: implications in clinical practice. Clin Chem Lab Med. 2005; 43:1215–7.
Article12. Hol EM, Pekny M. Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr Opin Cell Biol. 2015; 32:121–30.
Article13. Niebrój-Dobosz I, Rafałowska J, Lukasiuk M, Pfeffer A, Mossakowski MJ. Immunochemical analysis of some proteins in cerebrospinal fluid and serum of patients with ischemic strokes. Folia Neuropathol. 1994; 32:129–37.14. Herrmann M, Vos P, Wunderlich MT, de Bruijn CH, Lamers KJ. Release of glial tissue-specific proteins after acute stroke: a comparative analysis of serum concentrations of protein S-100B and glial fibrillary acidic protein. Stroke. 2000; 31:2670–7.
Article15. Dvorak F, Haberer I, Sitzer M, Foerch C. Characterisation of the diagnostic window of serum glial fibrillary acidic protein for the differentiation of intracerebral haemorrhage and ischaemic stroke. Cerebrovasc Dis. 2009; 27:37–41.
Article16. Kaneko T, Kasaoka S, Miyauchi T, Fujita M, Oda Y, Tsuruta R, et al. Serum glial fibrillary acidic protein as a predictive biomarker of neurological outcome after cardiac arrest. Resuscitation. 2009; 80:790–4.
Article17. Hayashida H, Kaneko T, Kasaoka S, Oshima C, Miyauchi T, Fujita M, et al. Comparison of the predictability of neurological outcome by serum procalcitonin and glial fibrillary acidic protein in postcardiac-arrest patients. Neurocrit Care. 2010; 12:252–7.
Article18. Wiesmann M, Steinmeier E, Magerkurth O, Linn J, Gottmann D, Missler U. Outcome prediction in traumatic brain injury: comparison of neurological status, CT findings, and blood levels of S100B and GFAP. Acta Neurol Scand. 2010; 121:178–85.
Article19. Pelinka LE, Kroepfl A, Schmidhammer R, Krenn M, Buchinger W, Redl H, et al. Glial fibrillary acidic protein in serum after traumatic brain injury and multiple trauma. J Trauma. 2004; 57:1006–12.
Article20. Pelinka LE, Kroepfl A, Leixnering M, Buchinger W, Raabe A, Redl H. GFAP versus S100B in serum after traumatic brain injury: relationship to brain damage and outcome. J Neurotrauma. 2004; 21:1553–61.
Article21. Nylén K, Ost M, Csajbok LZ, Nilsson I, Blennow K, Nellgård B, et al. Increased serum-GFAP in patients with severe traumatic brain injury is related to outcome. J Neurol Sci. 2006; 240:85–91.
Article22. Fraser DD, Close TE, Rose KL, Ward R, Mehl M, Farrell C, et al. Severe traumatic brain injury in children elevates glial fibrillary acidic protein in cerebrospinal fluid and serum. Pediatr Crit Care Med. 2011; 12:319–24.
Article23. Amalia L. Glial fibrillary acidic protein (GFAP): neuroinflammation biomarker in acute ischemic stroke. J Inflamm Res. 2021; 14:7501–6.
Article24. Kapoor S, Ahmad SA, Muquit S, Gusdon A, Khanduja S, Ziai W, et al. Brain injury plasma biomarkers in patients on VA-ECMO: a pilot prospective observational study. Preprints.org [Preprint]. 2023 [cited 2023 Nov 20]. Available from: https://doi.org/10.20944/preprints202306.1266.v1.25. Avila J, Lucas JJ, Perez M, Hernandez F. Role of tau protein in both physiological and pathological conditions. Physiol Rev. 2004; 84:361–84.
Article26. Gao Y, Tan L, Yu JT, Tan L. Tau in Alzheimer’s disease: mechanisms and therapeutic strategies. Curr Alzheimer Res. 2018; 15:283–300.
Article27. Zuckerman SL, Brett BL, Jeckell A, Yengo-Kahn AM, Solomon GS. Chronic traumatic encephalopathy and neurodegeneration in contact sports and American football. J Alzheimers Dis. 2018; 66:37–55.
Article28. Magnoni S, Esparza TJ, Conte V, Carbonara M, Carrabba G, Holtzman DM, et al. Tau elevations in the brain extracellular space correlate with reduced amyloid-β levels and predict adverse clinical outcomes after severe traumatic brain injury. Brain. 2012; 135(Pt 4):1268–80.
Article29. De Vos A, Bjerke M, Brouns R, De Roeck N, Jacobs D, Van den Abbeele L, et al. Neurogranin and tau in cerebrospinal fluid and plasma of patients with acute ischemic stroke. BMC Neurol. 2017; 17:170.
Article30. Mattsson N, Zetterberg H, Nielsen N, Blennow K, Dankiewicz J, Friberg H, et al. Serum tau and neurological outcome in cardiac arrest. Ann Neurol. 2017; 82:665–75.
Article31. Gabbita SP, Scheff SW, Menard RM, Roberts K, Fugaccia I, Zemlan FP. Cleaved-tau: a biomarker of neuronal damage after traumatic brain injury. J Neurotrauma. 2005; 22:83–94.
Article32. Gaetani L, Blennow K, Calabresi P, Di Filippo M, Parnetti L, Zetterberg H. Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry. 2019; 90:870–81.
Article33. Gafson AR, Barthélemy NR, Bomont P, Carare RO, Durham HD, Julien JP, et al. Neurofilaments: neurobiological foundations for biomarker applications. Brain. 2020; 143:1975–98.
Article34. Thebault S, Booth RA, Freedman MS. Blood neurofilament light chain: the neurologist’s troponin? Biomedicines. 2020; 8:523.
Article35. Moseby-Knappe M, Mattsson N, Nielsen N, Zetterberg H, Blennow K, Dankiewicz J, et al. Serum neurofilament light chain for prognosis of outcome after cardiac arrest. JAMA Neurol. 2019; 76:64–71.
Article36. Wihersaari L, Reinikainen M, Furlan R, Mandelli A, Vaahersalo J, Kurola J, et al. Neurofilament light compared to neuron-specific enolase as a predictor of unfavourable outcome after out-of-hospital cardiac arrest. Resuscitation. 2022; 174:1–8.
Article37. Levin H, Lybeck A, Frigyesi A, Arctaedius I, Thorgeirsdóttir B, Annborn M, et al. Plasma neurofilament light is a predictor of neurological outcome 12 h after cardiac arrest. Crit Care. 2023; 27:74.38. Martucci G, Arcadipane A, Tuzzolino F, Occhipinti G, Panarello G, Carcione C, et al. Identification of a circulating miRNA signature to stratify acute respiratory distress syndrome patients. J Pers Med. 2020; 11:15.
Article39. Zhang JM, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin. 2007; 45:27–37.
Article40. Datzmann T, Träger K. Extracorporeal membrane oxygenation and cytokine adsorption. J Thorac Dis. 2018; 10(Suppl 5):S653–60.
Article41. Caprarola SD, Ng DK, Carroll MK, Tekes A, Felling RJ, Salorio CF, et al. Pediatric ECMO: unfavorable outcomes are associated with inflammation and endothelial activation. Pediatr Res. 2022; 92:549–56.
Article42. Burrell AJC, Lubnow M, Enger TB, Nanjayya VB, Philipp A, Malfertheiner MV, et al. The impact of venovenous extracorporeal membrane oxygenation on cytokine levels in patients with severe acute respiratory distress syndrome: a prospective, observational study. Crit Care Resusc. 2017; 19(Suppl 1):37–44.43. Setiadi H, El Banayosy AM, Koerner MM, Maybauer MO, Harper MD, Horstmanshof DA, et al. Oncostatin M as a biomarker to predict the outcome of VV ECMO supported patients with acute pulmonary failure. J Heart Lung Transplant. 2020; 39(4 Supplement):S390.
Article44. Guo S, Li ZZ, Gong J, Xiang M, Zhang P, Zhao GN, et al. Oncostatin M confers neuroprotection against ischemic stroke. J Neurosci. 2015; 35:12047–62.
Article45. Knight D. Oncostatin M. In: Laurent GJ, Shapiro SD, editors. Encyclopedia of respiratory medicine. 2006; Academic Press. p. 254-8.46. Harrington JS, Huh JW, Schenck EJ, Nakahira K, Siempos II, Choi AM. Circulating mitochondrial DNA as predictor of mortality in critically ill patients: a systematic review of clinical studies. Chest. 2019; 156:1120–36.
Article47. Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012; 36:401–14.
Article48. Nakahira K, Hisata S, Choi AM. The roles of mitochondrial damage-associated molecular patterns in diseases. Antioxid Redox Signal. 2015; 23:1329–50.
Article49. Mao JY, Li DK, Zhang HM, Wang XT, Liu DW. Plasma mitochondrial DNA levels are associated with acute lung injury and mortality in septic patients. BMC Pulm Med. 2021; 21:66.
Article50. Puskarich MA, Shapiro NI, Trzeciak S, Kline JA, Jones AE. Plasma levels of mitochondrial DNA in patients presenting to the emergency department with sepsis. Shock. 2012; 38:337–40.
Article51. Fan JX, Zeng L, Chen L, Li Y, Pu H, Shen J, et al. The hidden link between plasma mitochondrial DNA level and cardiac dysfunction after cardiopulmonary bypass. Research Square [Preprint]. 2020 [cited 2023 Nov 20]. Available from: https://doi.org/10.21203/rs.3.rs-36879/v1.52. Bynum J, Meyer A, Darlington DN, McIntosh C, Peltier G, Taylor AS, et al. Increased mitochondrial DNA in an ECMO model is associated with loss of platelet function. Blood. 2017; 130(Suppl 1):1126.53. Tsai NW, Lin TK, Chen SD, Chang WN, Wang HC, Yang TM, et al. The value of serial plasma nuclear and mitochondrial DNA levels in patients with acute ischemic stroke. Clin Chim Acta. 2011; 412:476–9.
Article54. Corcoran RB, Chabner BA. Application of cell-free DNA analysis to cancer treatment. N Engl J Med. 2018; 379:1754–65.
Article55. Bustamante A, Mancha F, Macher HC, García-Berrocoso T, Giralt D, Ribó M, et al. Circulating cell-free DNA is a predictor of short-term neurological outcome in stroke patients treated with intravenous thrombolysis. J Circ Biomark. 2016; 5:1849454416668791.
Article56. Vajpeyee A, Wijatmiko T, Vajpeyee M, Taywade O, Pandey S, Chauhan PS. Clinical usefulness of cell-free DNA as a prognostic marker in acute ischemic stroke. Neurologist. 2020; 25:11–3.
Article57. Rainer TH, Wong LK, Lam W, Yuen E, Lam NY, Metreweli C, et al. Prognostic use of circulating plasma nucleic acid concentrations in patients with acute stroke. Clin Chem. 2003; 49:562–9.
Article58. Wang HC, Lin YJ, Lin WC, Ho JT, Chen WF, Chang WN, et al. The value of serial plasma nuclear and mitochondrial DNA levels in acute spontaneous intra-cerebral haemorrhage. Eur J Neurol. 2012; 19:1532–8.59. Hogan SR, Phan JH, Alvarado-Velez M, Wang MD, Bellamkonda RV, Fernández FM, et al. Discovery of lipidome alterations following traumatic brain injury via high-resolution metabolomics. J Proteome Res. 2018; 17:2131–43.
Article60. Gier EC, Pulliam AN, Gaul DA, Moore SG, LaPlaca MC, Fernández FM. Lipidome alterations following mild traumatic brain injury in the rat. Metabolites. 2022; 12:150.
Article61. Thomas I, Dickens AM, Posti JP, Czeiter E, Duberg D, Sinioja T, et al. Serum metabolome associated with severity of acute traumatic brain injury. Nat Commun. 2022; 13:2545.62. Gusdon AM, Savarraj JP, Redell JB, Paz A, Hinds S, Burkett A, et al. Lysophospholipids are associated with outcomes in hospitalized patients with mild traumatic brain injury. J Neurotrauma 2023 Oct 20 [Epub]. https://doi.org/10.1089/neu.2023.0046.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Extracorporeal Membrane Oxygenation for the Support of a Potential Organ Donor with a Fatal Brain Injury before Brain Death Determination
- Successful Retrieval of a Fractured Guidewire during Extracorporeal Membrane Oxygenator Insertion
- Catastrophic catecholamine-induced cardiomyopathy rescued by extracorporeal membrane oxygenation in recurrent malignant pheochromocytoma
- A case of rescuing a patient with acute cardiovascular instability from sudden and massive intraoperative pulmonary thromboembolism by extracorporeal membrane oxygenation
- Simultaneous Extracorporeal Membrane Oxygenation, Renal Replacement Therapy, and Plasma Exchange for Thrombocytopenia-Associated Multiple Organ Failure