J Pathol Transl Med.
2024 Jan;58(1):22-28. 10.4132/jptm.2023.11.21.
Identification of invasive subpopulations using spatial transcriptome analysis in thyroid follicular tumors
- Affiliations
-
- 1Department of Pathology, Graduate School of Medicine, Osaka University, Suita, Osaka, Japan
- 2Department of Diagnostic Pathology and Cytology, Kuma Hospital, Kobe, Hyogo, Japan
- 3Genome Information Research Center, Research Institute for Microbial Diseases, Osaka University, Suita, Osaka, Japan
- 4Institute for Open and Transdisciplinary Research Initiatives, Osaka University, Suita, Osaka, Japan
- KMID: 2550497
- DOI: http://doi.org/10.4132/jptm.2023.11.21
Abstract
- Background
Follicular tumors include follicular thyroid adenomas and carcinomas; however, it is difficult to distinguish between the two when the cytology or biopsy material is obtained from a portion of the tumor. The presence or absence of invasion in the resected material is used to differentiate between adenomas and carcinomas, which often results in the unnecessary removal of the adenomas. If nodules that may be follicular thyroid carcinomas are identified preoperatively, active surveillance of other nodules as adenomas is possible, which reduces the risk of surgical complications and the expenses incurred during medical treatment. Therefore, we aimed to identify biomarkers in the invasive subpopulation of follicular tumor cells.
Methods
We performed a spatial transcriptome analysis of a case of follicular thyroid carcinoma and examined the dynamics of CD74 expression in 36 cases.
Results
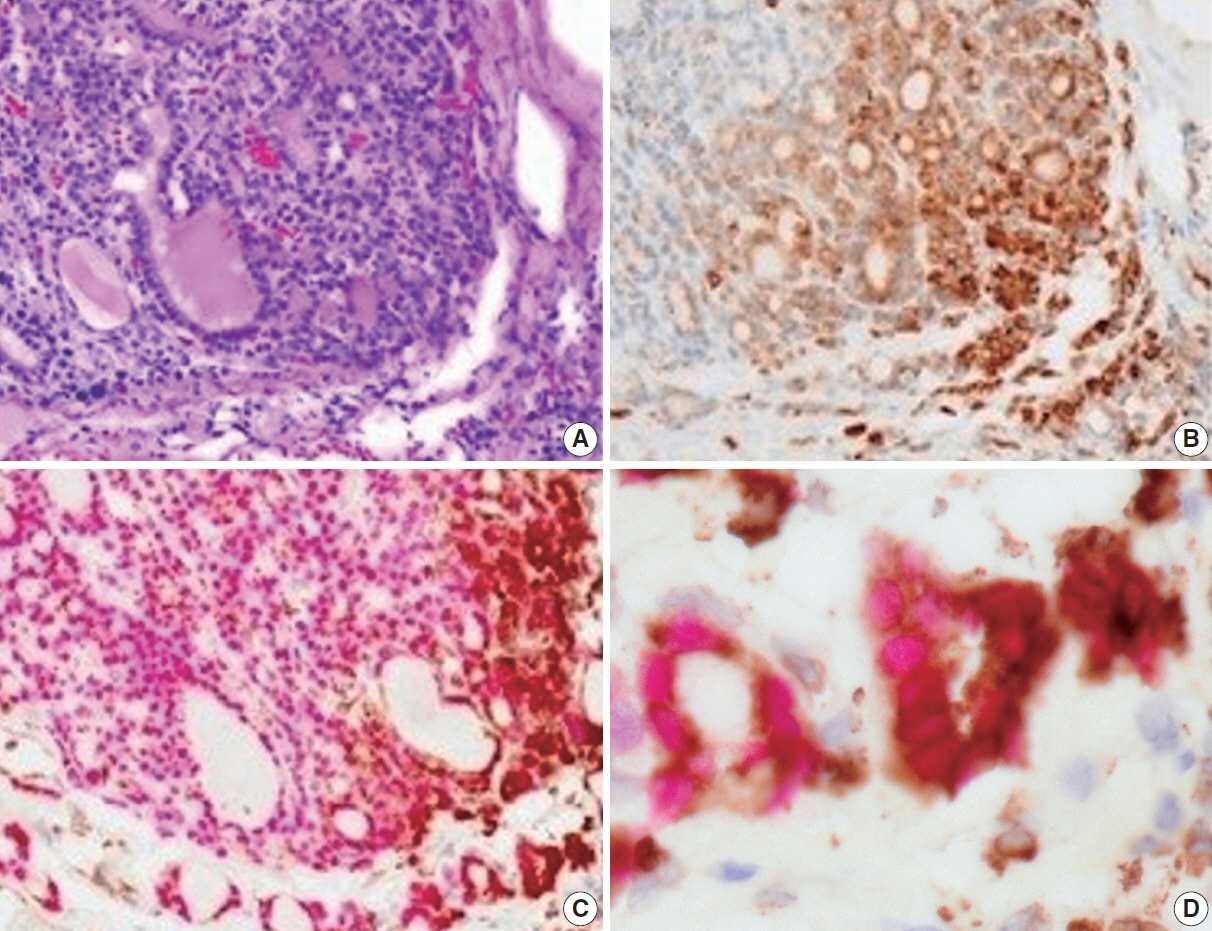
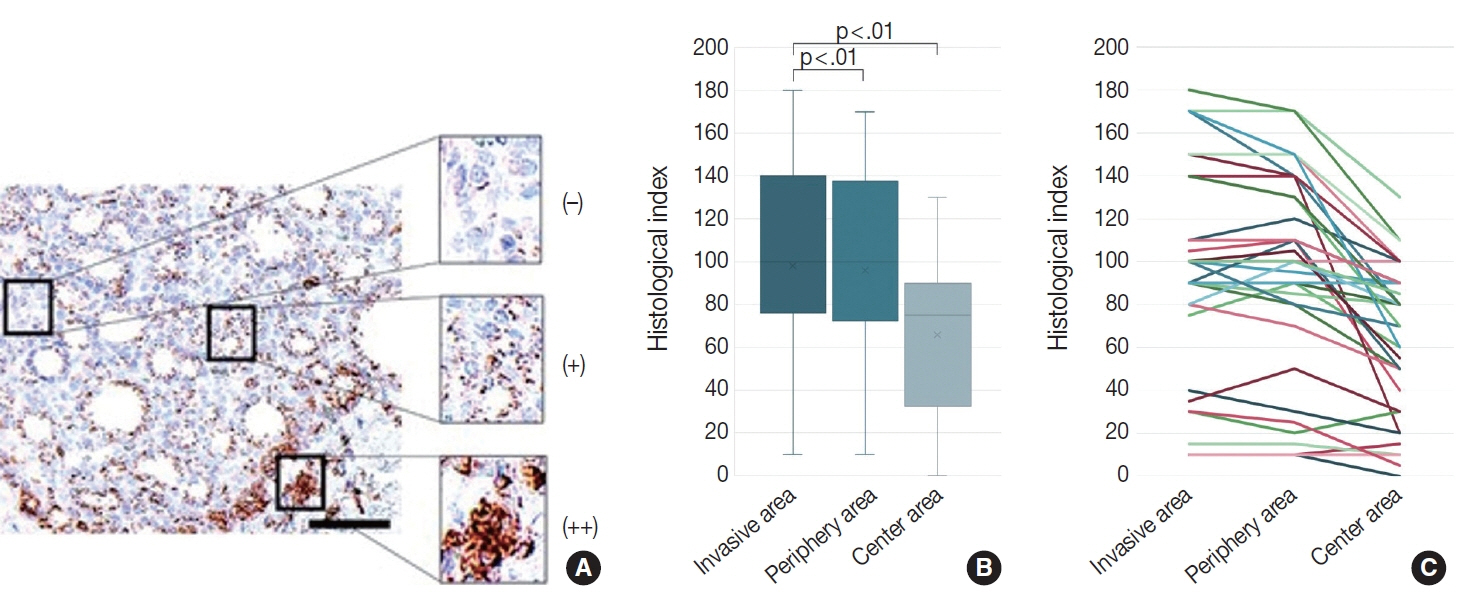
We identified a subpopulation in a region close to the invasive area, and this subpopulation expressed high levels of CD74. Immunohistochemically, CD74 was highly expressed in the invasive and peripheral areas of the tumor.
Conclusions
Although high CD74 expression has been reported in papillary and anaplastic thyroid carcinomas, it has not been analyzed in follicular thyroid carcinomas. Furthermore, the heterogeneity of CD74 expression in thyroid tumors has not yet been reported. The CD74-positive subpopulation identified in this study may be useful in predicting invasion of follicular thyroid carcinomas.
Figure
Reference
-
References
1. World Health Organization. WHO classification of tumours online. Endocrine and neuroendocrine tumours. 5th ed. Thyroid tumours [Internet]. Geneva: World Health Organization, 2022 [cited 2023 Oct 25]. Available from: https://tumourclassification.iarc.who.int/chapters/53.2. Auger M, Callegari F, Fadda G, Hirokawa M, Rooper L. Follicular neoplasm. In: Ali SZ, VanderLaan PA, eds. The Bethesda system for reporting thyroid cytopathology: definitions, criteria, and explanatory notes. In : Ali SZ, VanderLaan PA, editors. The Bethesda system for reporting thyroid cytopathology: definitions, criteria, and explanatory notes. 3rd ed. Cham: Springer;2023. p. 81–95.3. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016; 26:1–133.4. Hirokawa M, Suzuki A, Kawakami M, Kudo T, Miyauchi A. Criteria for follow-up of thyroid nodules diagnosed as follicular neoplasm without molecular testing: the experience of a high-volume thyroid centre in Japan. Diagn Cytopathol. 2022; 50:223–9.5. Sato A, Matsuda K, Motoyama T, et al. 53BP1 expression as a biomarker to differentiate thyroid follicular tumors. Endocr Connect. 2021; 10:309–15.6. Suzuki A, Hirokawa M, Furutate M, Hirai Y, Miyauchi A. LC-1000 Flow cytometry system improves risk stratification of thyroid nodules with suspected follicular neoplasm. JMA J. 2022; 5:124–6.7. Kim HK, Lee DW, Jin SY, Kim DW. Ki-67 labelling index and bax expression according to the capsular invasion in the follicular neoplasms of the thyroid. Korean J Pathol. 2001; 35:531–5.8. Kim CY, Cho SJ, Kim MK, Chae YS. SPARC expression in thyroid follicular adenomas and carcinomas. Korean J Pathol. 2000; 34:1016–21.9. Pujani M, Arora B, Pujani M, Singh SK, Tejwani N. Role of Ki-67 as a proliferative marker in lesions of thyroid. Indian J Cancer. 2010; 47:304–7.10. Mase T, Funahashi H, Koshikawa T, et al. HBME-1 immunostaining in thyroid tumors especially in follicular neoplasm. Endocr J. 2003; 50:173–7.11. Agustina H, Ahyati R, Suryanti S, Hernowo BS. The potential diagnostic value of Rac1 immunohistochemistry in follicular thyroid carcinoma. Malays J Pathol. 2022; 44:225–33.12. Cracolici V, Parilla M, Henriksen KJ, Cipriani NA. An evaluation of CD61 immunohistochemistry in identification of vascular invasion in follicular thyroid neoplasms. Head Neck Pathol. 2020; 14:399–405.13. Gracia Villacampa E, Larsson L, Mirzazadeh R, et al. Genome-wide spatial expression profiling in formalin-fixed tissues. Cell Genom. 2021; 1:100065.14. Watanabe R, Miura N, Kurata M, Kitazawa R, Kikugawa T, Saika T. Spatial gene expression analysis reveals characteristic gene expression patterns of de novo neuroendocrine prostate cancer coexisting with androgen receptor pathway prostate cancer. Int J Mol Sci. 2023; 24:8955.15. McInnes L, Healy J, Saul N, Grossberger L. UMAP: Uniform Manifold Approximation and Projection. J Open Source Softw. 2018; 3:861.16. Blondel VD, Guillaume JL, Lambiotte R, Lefebvre E. Fast unfolding of communities in large networks. J Stat Mech. 2008; 10:P10008.17. Schroder B. The multifaceted roles of the invariant chain CD74: more than just a chaperone. Biochim Biophys Acta. 2016; 1863:1269–81.18. Choi JW, Kim Y, Lee JH, Kim YS. CD74 expression is increased in high-grade, invasive urothelial carcinoma of the bladder. Int J Urol. 2013; 20:251–5.19. Hong WC, Lee DE, Kang HW, et al. CD74 promotes a pro-inflammatory tumor microenvironment by inducing S100A8 and S100A9 secretion in pancreatic cancer. Int J Mol Sci. 2023; 24:12993.20. Xu S, Li X, Tang L, Liu Z, Yang K, Cheng Q. CD74 correlated with malignancies and immune microenvironment in gliomas. Front Mol Biosci. 2021; 8:706949.21. Zeiner PS, Zinke J, Kowalewski DJ, et al. CD74 regulates complexity of tumor cell HLA class II peptidome in brain metastasis and is a positive prognostic marker for patient survival. Acta Neuropathol Commun. 2018; 6:18.22. Gai JW, Wahafu W, Song L, et al. Expression of CD74 in bladder cancer and its suppression in association with cancer proliferation, invasion and angiogenesis in HT-1376 cells. Oncol Lett. 2018; 15:7631–8.23. Wang P, Shi Q, Zuo T, He X, Yu J, Wang W. Expression of cluster of differentiation 74 in gallbladder carcinoma and the correlation with epithelial growth factor receptor levels. Oncol Lett. 2016; 11:2061–6.24. Liu Z, Chu S, Yao S, et al. CD74 interacts with CD44 and enhances tumorigenesis and metastasis via RHOA-mediated cofilin phosphorylation in human breast cancer cells. Oncotarget. 2016; 7:68303–13.25. Zhang JT, Zhang J, Wang SR, et al. Spatial downregulation of CD74 signatures may drive invasive component development in part-solid lung adenocarcinoma. iScience. 2023; 26:107699.26. Gold DV, Stein R, Burton J, Goldenberg DM. Enhanced expression of CD74 in gastrointestinal cancers and benign tissues. Int J Clin Exp Pathol. 2010; 4:1–12.27. Varinelli L, Caccia D, Volpi CC, et al. 4-IPP, a selective MIF inhibitor, causes mitotic catastrophe in thyroid carcinomas. Endocr Relat Cancer. 2015; 22:759–75.28. Cheng SP, Liu CL, Chen MJ, et al. CD74 expression and its therapeutic potential in thyroid carcinoma. Endocr Relat Cancer. 2015; 22:179–90.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pathologic basis of the sonographic differences between thyroid cancer and noninvasive follicular thyroid neoplasm with papillary-like nuclear features
- Understanding Neoplasm of Uncertain or Unknown Behavior of the Thyroid in Korean Clinical Practice
- Definition and Prognostic Factor of Minimally Invasive Follicular Thyroid Carcinoma
- Ki-67 Labelling Index and Bax Expression According to the Capsular Invasion in the Follicular Neoplasms of the Thyroid
- SPARC Expression in Thyroid Follicular Adenomas and Carcinomas