Cancer Res Treat.
2024 Jan;56(1):305-313. 10.4143/crt.2023.647.
Incidence and Features of Lymphoid Proliferation and Lymphomas after Solid Organ or Hematopoietic Stem Cell Transplantation in a National Database Cohort
- Affiliations
-
- 1Department of Pediatric Hematology-Oncology, Yonsei Cancer Center, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 2Biostatistics Collaboration Unit, Department of Biomedical Systems Informatics, Yonsei University College of Medicine, Seoul, Korea
- 3Division of Infectious Diseases, Department of Internal Medicine, Inje University Ilsan Paik Hospital, Goyang, Korea
- 4Department of Pediatrics, Hanyang University Hospital, Hanyang University College of Medicine, Seoul, Korea
- 5Department of Pediatrics, Severance Children’s Hospital, Yonsei University College of Medicine, Seoul, Korea
- 6Institute for Immunology and Immunological Diseases, Yonsei University College of Medicine, Seoul, Korea
- 7Department of Surgery, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 8Division of Biostatistics, Department of Biomedical Systems Informatics, Yonsei University College of Medicine, Seoul, Korea
- 9Division of Pediatric Surgery, Severance Children’s Hospital, Department of Surgery, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2550345
- DOI: http://doi.org/10.4143/crt.2023.647
Abstract
- Purpose
Post-transplantation lymphoproliferative disorders (PTLDs) after hematopoietic stem transplantation (HCT) or solid organ transplantation (SOT) result in poorer outcomes, including death. There are limited large cohort data on the incidence and natural course of PTLD in Asians.
Materials and Methods
We investigated PTLD using Korean national health insurance claims data of 47,518 patients who underwent HCT or SOT in 2008-2020. Patient demographics, time and type of PTLD diagnosis, type of PTLD treatment, and death data were collected. We used Fine and Gray subdistribution hazard models to calculate the cumulative incidence and risk factors for PTLD.
Results
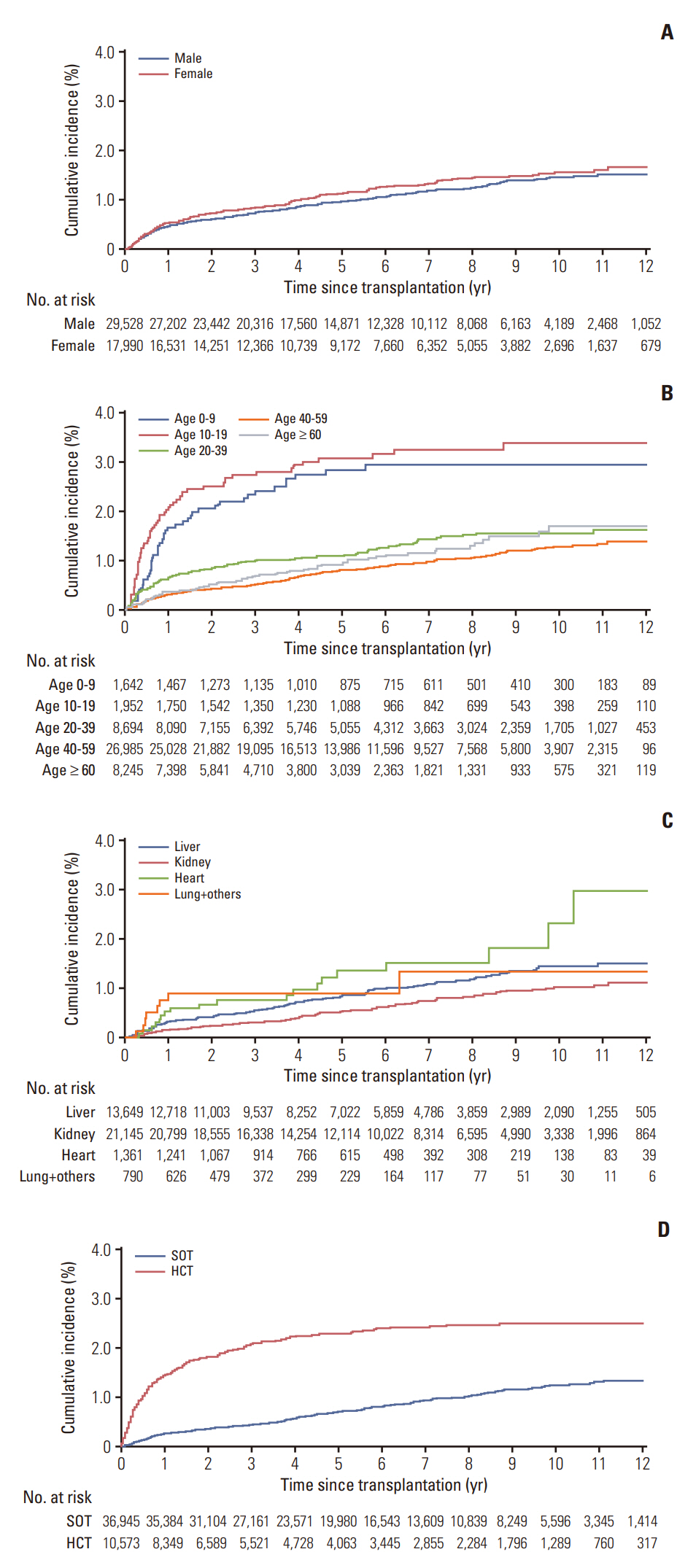
During median follow-up of 5.32 years, PTLD occurred in 294 of 36,945 SOT patients (0.79%) and 235 of 10,573 HCT patients (2.22%). Cumulative incidence of PTLD were 0.49% at 1 year, 1.02% at 5 years, and 1.50% at 10 years post-transplantation. Age < 20 years (subdistribution hazard ratio [SHR] of 1.67 in age 10-19, SHR 1.51 in age 0-9), HCT (SHR 3.02), heart transplantation (SHR 2.27), and liver transplantation (SHR 1.47) were significant risk factors for PTLD. The presence of PTLD was associated with an increased risk of death (hazard ratio of 2.84). Overall, 5-year survival of PTLD patients was 68.9% (95% confidence interval, 64.9 to 73.2).
Conclusion
We observed a steady increase in PTLD over 10 years after HCT or SOT in this large cohort study. Pediatric age group, HCT, liver transplantation, and heart transplantation were suggested to be risk factors for PTLD, and PTLD was associated with a higher risk of death.
Keyword
Figure
Reference
-
References
1. Singavi AK, Harrington AM, Fenske TS. Post-transplant lymphoproliferative disorders. Cancer Treat Res. 2015; 165:305–27.2. Dharnidharka VR, Webster AC, Martinez OM, Preiksaitis JK, Leblond V, Choquet S. Post-transplant lymphoproliferative disorders. Nat Rev Dis Primers. 2016; 2:15088.3. Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IB, Berti E, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia. 2022; 36:1720–48.4. Luskin MR, Heil DS, Tan KS, Choi S, Stadtmauer EA, Schuster SJ, et al. The impact of EBV status on characteristics and outcomes of post-transplantation lymphoproliferative disorder. Am J Transplant. 2015; 15:2665–73.5. Enok Bonong PR, Zahreddine M, Buteau C, Duval M, Laporte L, Lacroix J, et al. Factors associated with post-transplant active Epstein-Barr virus infection and lymphoproliferative disease in hematopoietic stem cell transplant recipients: a systematic review and meta-analysis. Vaccines (Basel). 2021; 9:288.6. Poeschel V, Held G, Ziepert M, Witzens-Harig M, Holte H, Thurner L, et al. Four versus six cycles of CHOP chemotherapy in combination with six applications of rituximab in patients with aggressive B-cell lymphoma with favourable prognosis (FLYER): a randomised, phase 3, non-inferiority trial. Lancet. 2019; 394:2271–81.7. Allen UD, Preiksaitis JK; AST Infectious Diseases Community of Practice. Post-transplant lymphoproliferative disorders, Epstein-Barr virus infection, and disease in solid organ transplantation: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019; 33:e13652.8. Chan TS, Hwang YY, Gill H, Au WY, Leung AY, Tse E, et al. Post-transplant lymphoproliferative diseases in Asian solid organ transplant recipients: late onset and favorable response to treatment. Clin Transplant. 2012; 26:679–83.9. Seo E, Kim J, Oh SH, Kim KM, Kim DY, Lee J. Epstein-Barr viral load monitoring for diagnosing post-transplant lymphoproliferative disorder in pediatric liver transplant recipients. Pediatr Transplant. 2020; 24:e13666.10. Liu Y, Sun LY, Zhu ZJ, Wei L, Qu W, Wang L, et al. Posttransplant lymphoproliferative disorder after paediatric liver transplantation. Int J Clin Pract. 2021; 75:e13843.11. Fujimoto A, Hiramoto N, Yamasaki S, Inamoto Y, Uchida N, Maeda T, et al. Risk factors and predictive scoring system for post-transplant lymphoproliferative disorder after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2019; 25:1441–9.12. Kim JA, Yoon S, Kim LY, Kim DS. Towards actualizing the value potential of Korea Health Insurance Review and Assessment (HIRA) data as a resource for health research: strengths, limitations, applications, and strategies for optimal use of HIRA Data. J Korean Med Sci. 2017; 32:718–28.13. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999; 94:496–509.14. Curtis RE, Travis LB, Rowlings PA, Socie G, Kingma DW, Banks PM, et al. Risk of lymphoproliferative disorders after bone marrow transplantation: a multi-institutional study. Blood. 1999; 94:2208–16.15. Landgren O, Gilbert ES, Rizzo JD, Socie G, Banks PM, Sobocinski KA, et al. Risk factors for lymphoproliferative disorders after allogeneic hematopoietic cell transplantation. Blood. 2009; 113:4992–5001.16. Styczynski J, Gil L, Tridello G, Ljungman P, Donnelly JP, van der Velden W, et al. Response to rituximab-based therapy and risk factor analysis in Epstein Barr Virus-related lymphoproliferative disorder after hematopoietic stem cell transplant in children and adults: a study from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Clin Infect Dis. 2013; 57:794–802.17. Sundin M, Le Blanc K, Ringden O, Barkholt L, Omazic B, Lergin C, et al. The role of HLA mismatch, splenectomy and recipient Epstein-Barr virus seronegativity as risk factors in post-transplant lymphoproliferative disorder following allogeneic hematopoietic stem cell transplantation. Haematologica. 2006; 91:1059–67.18. Pegoraro F, Favre C. Post-transplantation lymphoproliferative disorder after haematopoietic stem cell transplantation. Ann Hematol. 2021; 100:865–78.19. Santarsieri A, Rudge JF, Amin I, Gelson W, Parmar J, Pettit S, et al. Incidence and outcomes of post-transplant lymphoproliferative disease after 5365 solid-organ transplants over a 20-year period at two UK transplant centres. Br J Haematol. 2022; 197:310–9.20. Kasiske BL, Kukla A, Thomas D, Wood Ives J, Snyder JJ, Qiu Y, et al. Lymphoproliferative disorders after adult kidney transplant: epidemiology and comparison of registry report with claims-based diagnoses. Am J Kidney Dis. 2011; 58:971–80.21. Francis A, Johnson DW, Teixeira-Pinto A, Craig JC, Wong G. Incidence and predictors of post-transplant lymphoproliferative disease after kidney transplantation during adulthood and childhood: a registry study. Nephrol Dial Transplant. 2018; 33:881–9.22. Sprangers B, Riella LV, Dierickx D. Posttransplant lymphoproliferative disorder following kidney transplantation: a review. Am J Kidney Dis. 2021; 78:272–81.23. Caillard S, Lamy FX, Quelen C, Dantal J, Lebranchu Y, Lang P, et al. Epidemiology of posttransplant lymphoproliferative disorders in adult kidney and kidney pancreas recipients: report of the French registry and analysis of subgroups of lymphomas. Am J Transplant. 2012; 12:682–93.24. Narkewicz MR, Green M, Dunn S, Millis M, McDiarmid S, Mazariegos G, et al. Decreasing incidence of symptomatic Epstein-Barr virus disease and posttransplant lymphoproliferative disorder in pediatric liver transplant recipients: report of the studies of pediatric liver transplantation experience. Liver Transpl. 2013; 19:730–40.25. Opelz G, Dohler B. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant. 2004; 4:222–30.26. Engels EA, Pfeiffer RM, Fraumeni JF Jr, Kasiske BL, Israni AK, Snyder JJ, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011; 306:1891–901.27. Kitchlu A, Dixon S, Dirk JS, Chanchlani R, Vasilevska-Ristovska J, Borges K, et al. Elevated risk of cancer after solid organ transplant in childhood: a population-based cohort study. Transplantation. 2019; 103:588–96.28. Annual report of cancer statistics in Korea in 2019 [Internet]. Goyan: National Cancer Center;2019. [cited 2023 Feb 28]. Available from: https://www.ncc.re.kr/cancerStatsView.ncc?bbsnum=598.29. Kim SK, Choi JS, Kim D, Kang CI, Chung DR, Peck KR, et al. Analysis of the change in seropositive rate of Epstein-Barr virus in Koreans: a single-center study. Pediatr Infect Vaccine. 2020; 27:117–26.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Opening the era of in vivo xenotransplantation model for hematopoietic stem cell transplantation
- Hematopoietic Stem Cell Transplantation
- Hematopoietic stem cell transplantation: overview for general pediatrician
- Cell Therapy in Hematopoietic Stem Cell Transplantation
- Current Trends and Prospect of Cell Therapy using Hematopoietic Stem Cells