Cancer Res Treat.
2024 Jan;56(1):37-47. 10.4143/crt.2023.433.
A Phase II Trial of Nintedanib in Patients with Metastatic or Recurrent Head and Neck Squamous Cell Carcinoma: In-Depth Analysis of Nintedanib Arm from the KCSG HN 15-16 TRIUMPH Trial
- Affiliations
-
- 1Divison of Medical Oncology, Department of Internal Medicine, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea
- 2Division of Medical Oncology, Department of Internal Medicine, Gachon University Gil Medical Center, Incheon, Korea
- 3Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- 4Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 5Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 6Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea
- 7Department of Hematology-Oncology, Ajou University School of Medicine, Suwon, Korea
- 8Division of Medical Oncology, Department of Internal Medicine, St. Vincent's Hospital, College of Medicine, The Catholic University of Korea, Suwon, Korea
- 9Department of Internal Medicine, SMG-SNU Boramae Medical Center, Seoul, Korea
- KMID: 2550321
- DOI: http://doi.org/10.4143/crt.2023.433
Abstract
- Purpose
Precision oncology approach for recurrent and metastatic head and neck squamous cell carcinoma (HNSCC) is necessary due to its dismal prognosis. We performed a genomic profile-based umbrella trial of patients with platinum-refractory HNSCC (KCSG-TRIUMPH). Here, we present an in-depth report of the the nintedanib arm (arm 3) of the current trial.
Materials and Methods
The TRIUMPH study was a multicenter, open-label, single-arm phase 2 trial, in which patients were assigned to treatment arms based on next-generation sequencing (NGS)–based, matching genomic profiles. Patients whose tumors harbor fibroblast growth factor receptor (FGFR) alteration were enrolled in the nintedanib arm (arm 3) as part of the TRIUMPH study. The primary endpoint was the overall response rate (ORR), and secondary endpoints included overall survival (OS), progression-free survival (PFS), safety, and biomarker analysis.
Results
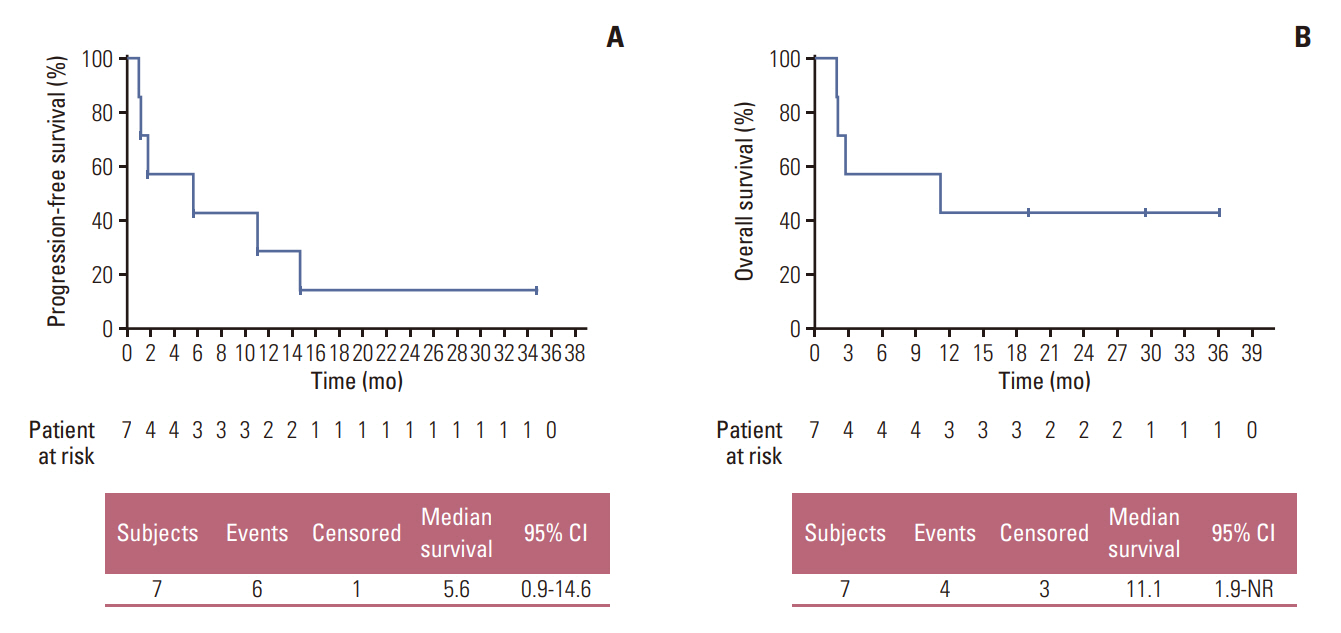
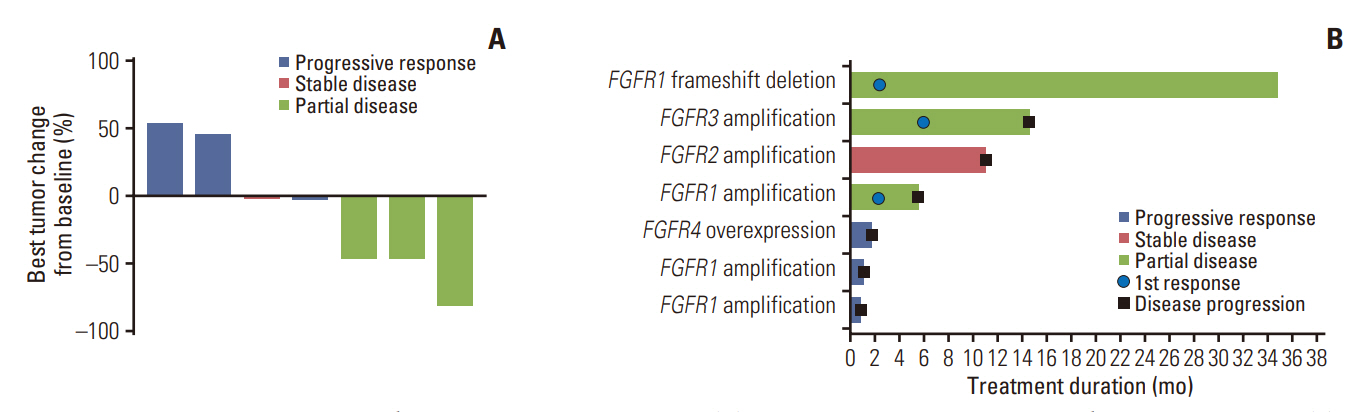
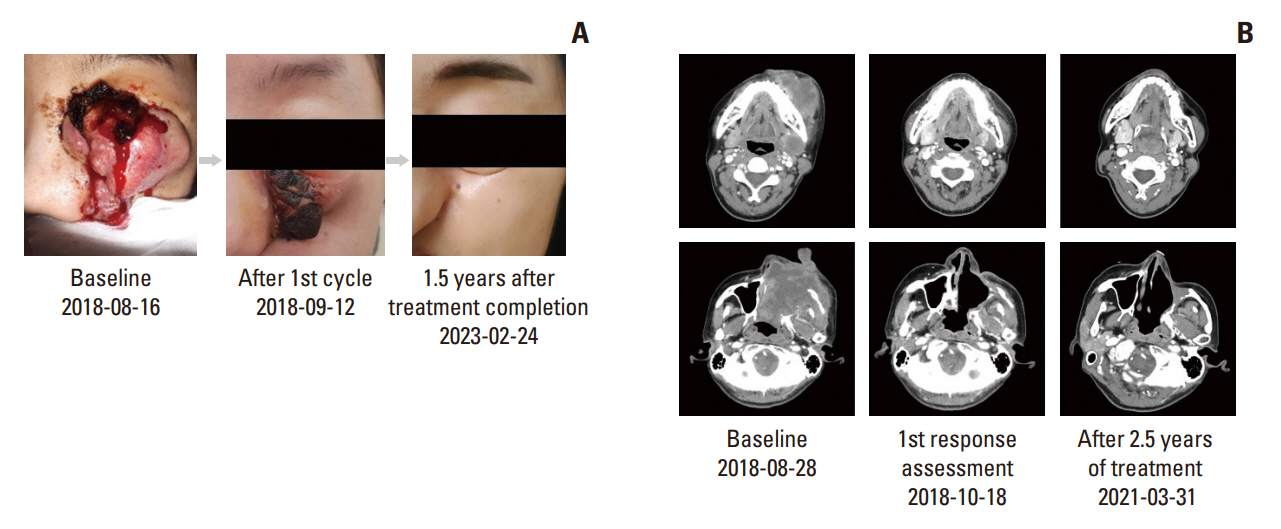
Between October 2017 and August 2020, 207 were enrolled in the TRIUMPH study, and eight were enrolled in the nintedanib arm. ORR and disease control rate were 42.9% and 57.1%, respectively. The median PFS was 5.6 months and the median duration of response was 9.1 months. Median OS was 11.1 months. One patient maintained the partial response for 36 months. Overall, the toxicity profiles were manageable.
Conclusion
Single-agent nintedanib has demonstrated significant efficacy in FGFR-mutated, recurrent or metastatic HNSCC patients, with tolerable toxicity profiles. The results from the study have provided the basis for routine NGS screening and FGFR-targeted therapy. Because of the small number of patients due to slow accrual in this study, further studies with a larger cohort are warranted for statistical power.
Keyword
Figure
Reference
-
References
1. Mody MD, Rocco JW, Yom SS, Haddad RI, Saba NF. Head and neck cancer. Lancet. 2021; 398:2289–99.2. Vermorken JB, Trigo J, Hitt R, Koralewski P, Diaz-Rubio E, Rolland F, et al. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol. 2007; 25:2171–7.3. Morris LG, Chandramohan R, West L, Zehir A, Chakravarty D, Pfister DG, et al. The molecular landscape of recurrent and metastatic head and neck cancers: insights from a precision oncology sequencing platform. JAMA Oncol. 2017; 3:244–55.4. Marret G, Bieche I, Dupain C, Borcoman E, du Rusquec P, Ricci F, et al. Genomic alterations in head and neck squamous cell carcinoma: level of evidence according to ESMO Scale for Clinical Actionability of Molecular Targets (ESCAT). JCO Precis Oncol. 2021; 5:215–26.5. Keam B, Hong MH, Shin SH, Heo SG, Kim JE, Ahn HK, et al. Personalized biomarker-based umbrella trial for patients with recurrent or metastatic head and neck squamous cell carcinoma: KCSG HN 15-16 TRIUMPH trial. J Clin Oncol. 2023; Sep. 12. [Epub]. https://doi.org/10.1200/JCO.22.02786.6. Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015; 517:576–82.7. Wang Z, Anderson KS. Therapeutic targeting of FGFR signaling in head and neck cancer. Cancer J. 2022; 28:354–62.8. Seiwert TY, Zuo Z, Keck MK, Khattri A, Pedamallu CS, Stricker T, et al. Integrative and comparative genomic analysis of HPV-positive and HPV-negative head and neck squamous cell carcinomas. Clin Cancer Res. 2015; 21:632–41.9. Roth GJ, Binder R, Colbatzky F, Dallinger C, Schlenker-Herceg R, Hilberg F, et al. Nintedanib: from discovery to the clinic. J Med Chem. 2015; 58:1053–63.10. Hilberg F, Roth GJ, Krssak M, Kautschitsch S, Sommergruber W, Tontsch-Grunt U, et al. BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res. 2008; 68:4774–82.11. Reck M, Kaiser R, Mellemgaard A, Douillard JY, Orlov S, Krzakowski M, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated nonsmall-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol. 2014; 15:143–55.12. Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014; 370:2071–82.13. Hanna NH, Kaiser R, Sullivan RN, Aren OR, Ahn MJ, Tiangco B, et al. Nintedanib plus pemetrexed versus placebo plus pemetrexed in patients with relapsed or refractory, advanced non-small cell lung cancer (LUME-Lung 2): a randomized, double-blind, phase III trial. Lung Cancer. 2016; 102:65–73.14. McCormack PL. Nintedanib: first global approval. Drugs. 2015; 75:129–39.15. Lim SM, Cho SH, Hwang IG, Choi JW, Chang H, Ahn MJ, et al. Investigating the feasibility of targeted next-generation sequencing to guide the treatment of head and neck squamous cell carcinoma. Cancer Res Treat. 2019; 51:300–12.16. Joshi NA, Fass JN. Sickle: a sliding-window, adaptive, quality-based trimming tool for FastQ files (version 1.33) [Internet]. San Francisco, CA: GitHub Inc;2011. [cited 2023 Mar 1]. Available from: https://github.com/najoshi/sickle.17. Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009; 25:1754–60.18. McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010; 20:1297–303.19. Otsubo K, Kishimoto J, Ando M, Kenmotsu H, Minegishi Y, Horinouchi H, et al. Nintedanib plus chemotherapy for nonsmall cell lung cancer with idiopathic pulmonary fibrosis: a randomised phase 3 trial. Eur Respir J. 2022; 60:2200380.20. Ou J, Zhu LJ. trackViewer: a Bioconductor package for interactive and integrative visualization of multi-omics data. Nat Methods. 2019; 16:453–4.21. Conley BA, Staudt L, Takebe N, Wheeler DA, Wang L, Cardenas MF, et al. The exceptional responders initiative: feasibility of a National Cancer Institute pilot study. J Natl Cancer Inst. 2021; 113:27–37.22. Helsten T, Elkin S, Arthur E, Tomson BN, Carter J, Kurzrock R. The FGFR landscape in cancer: analysis of 4,853 tumors by next-generation sequencing. Clin Cancer Res. 2016; 22:259–67.23. Hussain SA, Lester JF, Jackson R, Gornall M, Qureshi M, Elliott A, et al. Addition of nintedanib or placebo to neoadjuvant gemcitabine and cisplatin in locally advanced muscle-invasive bladder cancer (NEOBLADE): a double-blind, randomised, phase 2 trial. Lancet Oncol. 2022; 23:650–8.24. Barra F, Lagana AS, Ghezzi F, Casarin J, Ferrero S. Nintedanib for advanced epithelial ovarian cancer: a change of perspective? Summary of evidence from a systematic review. Gynecol Obstet Invest. 2019; 84:107–17.25. Ray-Coquard I, Cibula D, Mirza MR, Reuss A, Ricci C, Colombo N, et al. Final results from GCIG/ENGOT/AGO-OVAR 12, a randomised placebo-controlled phase III trial of nintedanib combined with chemotherapy for newly diagnosed advanced ovarian cancer. Int J Cancer. 2020; 146:439–48.26. Kim Y, Lee SJ, Lee JY, Lee SH, Sun JM, Park K, et al. Clinical trial of nintedanib in patients with recurrent or metastatic salivary gland cancer of the head and neck: a multicenter phase 2 study (Korean Cancer Study Group HN14-01). Cancer. 2017; 123:1958–64.27. Pearson A, Smyth E, Babina IS, Herrera-Abreu MT, Tarazona N, Peckitt C, et al. High-level clonal FGFR amplification and response to FGFR inhibition in a translational clinical trial. Cancer Discov. 2016; 6:838–51.28. Loriot Y, Necchi A, Park SH, Garcia-Donas J, Huddart R, Burgess E, et al. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N Engl J Med. 2019; 381:338–48.29. Abou-Alfa GK, Sahai V, Hollebecque A, Vaccaro G, Melisi D, Al-Rajabi R, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020; 21:671–84.30. Javle M, Roychowdhury S, Kelley RK, Sadeghi S, Macarulla T, Weiss KH, et al. Infigratinib (BGJ398) in previously treated patients with advanced or metastatic cholangiocarcinoma with FGFR2 fusions or rearrangements: mature results from a multicentre, open-label, single-arm, phase 2 study. Lancet Gastroenterol Hepatol. 2021; 6:803–15.31. Goyal L, Meric-Bernstam F, Hollebecque A, Valle JW, Morizane C, Karasic TB, et al. Futibatinib for FGFR2-rearranged intrahepatic cholangiocarcinoma. N Engl J Med. 2023; 388:228–39.32. Ellinghaus P, Neureiter D, Nogai H, Stintzing S, Ocker M. Patient selection approaches in FGFR inhibitor trials: many paths to the same end? Cells. 2022; 11:3180.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of All-trans-retinoic Acid on the Cell Cycle of Head and Neck Squamous Cell Carcinomas
- The Patterns of Expression of p53, bcl-2 and Bax after Irradiation in Cell Lines of Squamous Cell Carcinoma of Head and Neck
- Pharmacological treatment of pulmonary fibrosis
- Clinical analysis of distant metastases in the squamous cell carcinoma of head and neck
- Phase II Trial of Combined Durvalumab Plus Tremelimumab with Proton Therapy for Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma