Korean J Transplant.
2023 Dec;37(4):250-259. 10.4285/kjt.23.0048.
Cell cycle arrest biomarkers for the early detection of acute allograft dysfunction and acute rejection in living donor kidney transplantation: a cross-sectional study from Egypt
- Affiliations
-
- 1Nephrology Unit, Department of Internal Medicine, Faculty of Medicine, Cairo University, Cairo, Egypt
- 2Nasser Institute for Research and Treatment, Ministry of Health, Cairo, Egypt
- KMID: 2550236
- DOI: http://doi.org/10.4285/kjt.23.0048
Abstract
- Background
Urinary tissue inhibitor of metalloproteinase-2 (TIMP-2) and insulin-like growth factor-binding protein 7 (IGFBP7) are G1 cell arrest biomarkers that have demonstrated accuracy and validity in predicting and diagnosing acute kidney injury (AKI). This study aimed to evaluate the validity of [TIMP-2]×[IGFBP7] in diagnosing acute allograft dysfunction and its utility in distinguishing acute rejection (AR) from nonrejection causes in kidney transplantation.
Methods
This study included 48 adult living donor kidney transplant recipients (KTRs; 18 with AR, 15 with nonrejection causes of AKI, and 15 with stable grafts). Urinary TIMP- 2 and IGFBP7 were measured, and [TIMP-2]×[IGFBP7] was calculated in all subjects.
Results
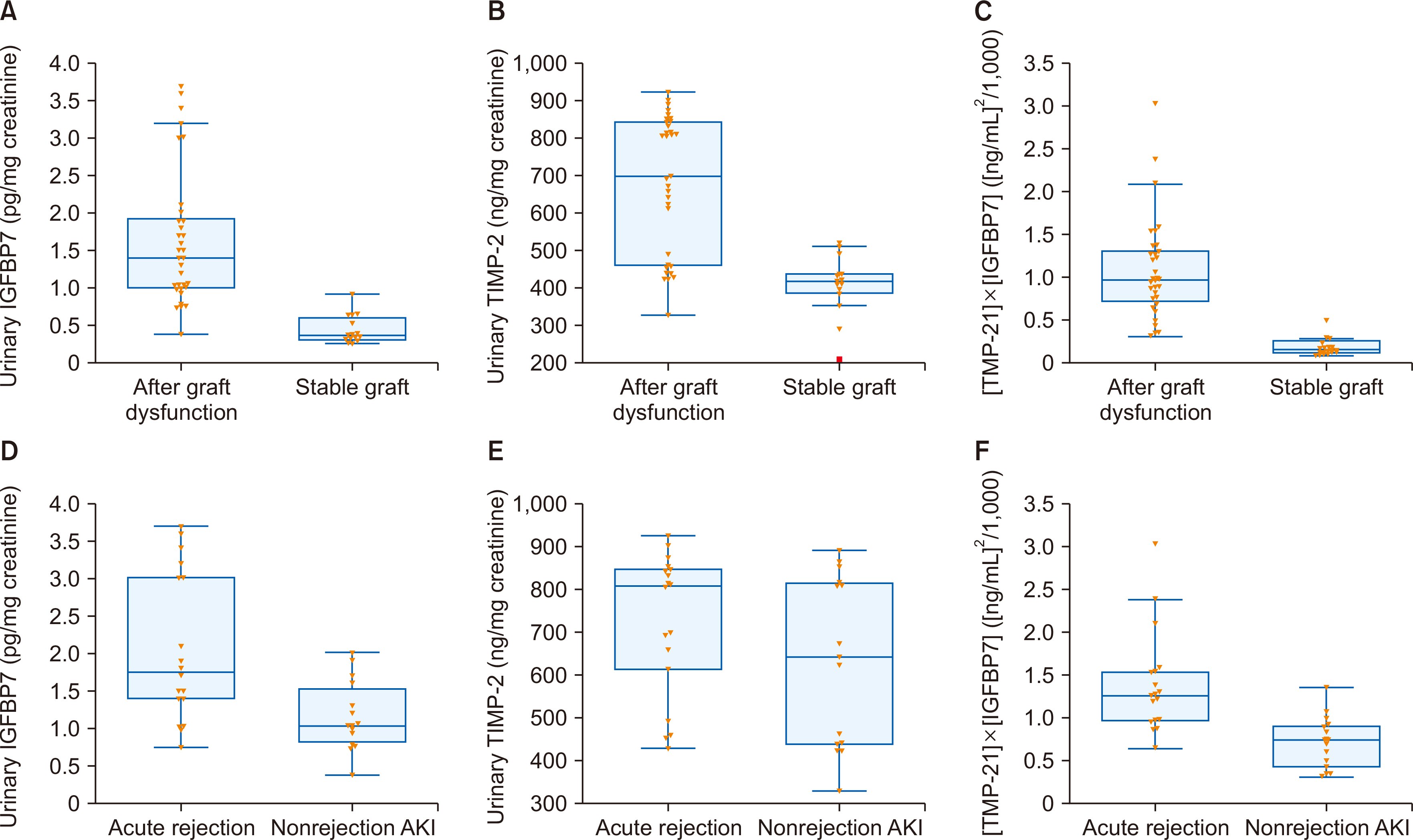
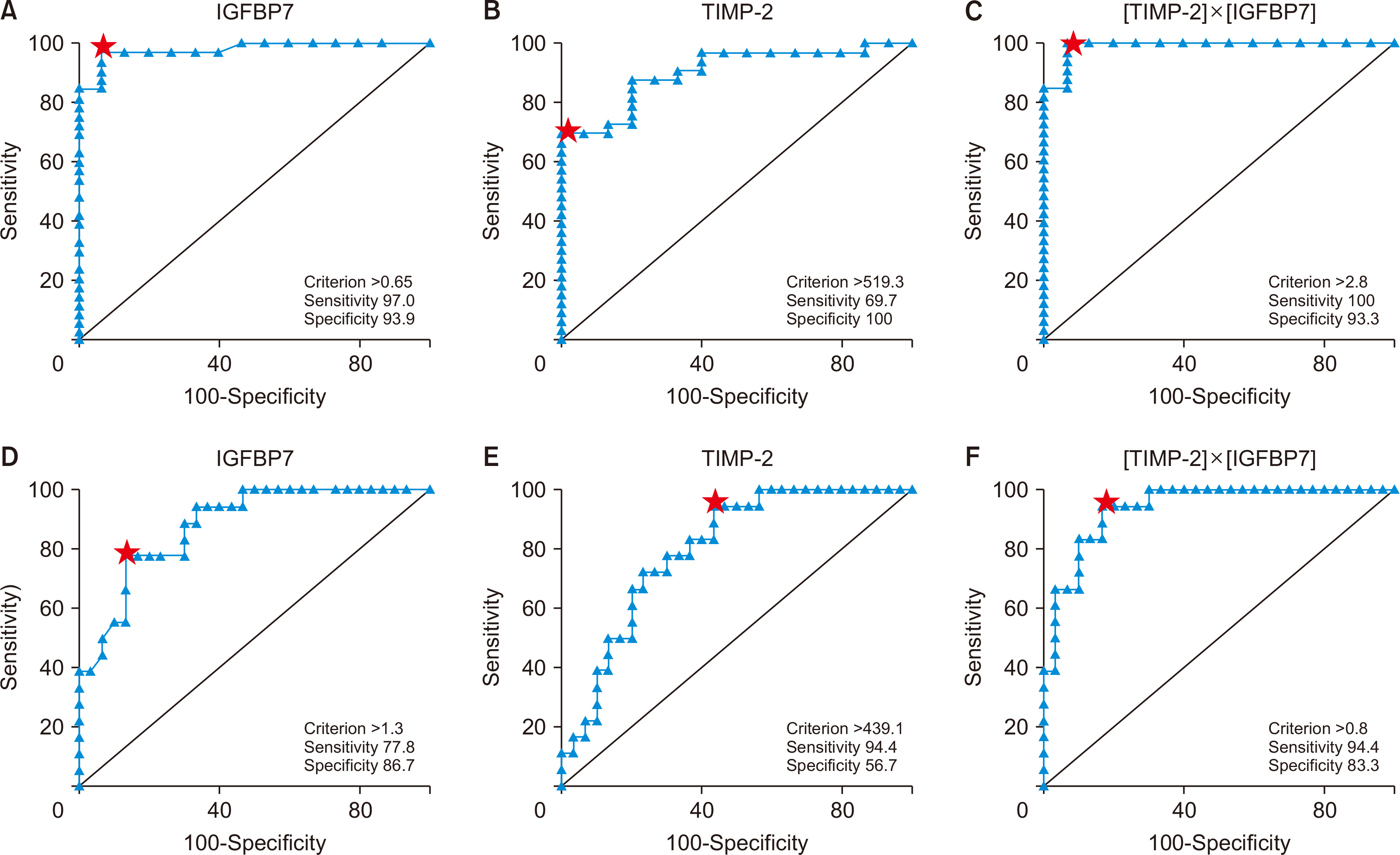
IGFBP7, TIMP-2, and [TIMP-2]×[IGFBP7] were statistically significantly higher in KTRs with acute allograft dysfunction than in those with stable grafts. [TIMP-2]×[IGFBP7] was statistically significantly higher in KTRs with AR than in those with nonrejection AKI. [TIMP-2]×[IGFBP7] at a cutoff level of 0.278 (ng/mL)2 /1,000 had an area under the curve (AUC) of 0.99 with a sensitivity of 100% and a specificity of 93.3% in diagnos-ing acute allograft dysfunction, while at a cutoff level of 0.803 (ng/mL)2 /1,000 had an AUC of 0.939 with a sensitivity of 94.4% and a specificity of 83.3% in diagnosing AR.
Conclusions
Besides its role in the early detection of acute allograft dysfunction, [TIMP-2]×[IGFBP7] may help to differentiate between AR and nonrejection causes in KTRs. However, whether and how urinary [TIMP-2]×[IGFBP7] can be used in clinical diagnosis still requires further research.
Keyword
Figure
Reference
-
1. Meier-Kriesche HU, Ojo AO, Hanson JA, Cibrik DM, Punch JD, Leichtman AB, et al. 2000; Increased impact of acute rejection on chronic allograft failure in recent era. Transplantation. 70:1098–100. DOI: 10.1097/00007890-200010150-00018. PMID: 11045649.2. Opelz G. the Collaborative Transplant Study. 1997; Critical evaluation of the association of acute with chronic graft rejection in kidney and heart transplant recipients. Transplant Proc. 29:73–6. DOI: 10.1016/S0041-1345(96)00013-9. PMID: 9123162.3. Tapia-Canelas C, Zometa R, López-Oliva MO, Jiménez C, Rivas B, Escuin F, et al. 2014; Complications associated with renal graft biopsy in transplant patients. Nefrologia. 34:115–9.4. Oh DJ. 2020; A long journey for acute kidney injury biomarkers. Ren Fail. 42:154–65. DOI: 10.1080/0886022X.2020.1721300. PMID: 32050834. PMCID: PMC7034110.5. Endre ZH, Pickering JW, Walker RJ, Devarajan P, Edelstein CL, Bonventre JV, et al. 2011; Improved performance of urinary biomarkers of acute kidney injury in the critically ill by stratification for injury duration and baseline renal function. Kidney Int. 79:1119–30. DOI: 10.1038/ki.2010.555. PMID: 21307838. PMCID: PMC3884688.6. Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. 2010; Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med. 16:535–43. DOI: 10.1038/nm.2144. PMID: 20436483. PMCID: PMC3928013.7. Emlet DR, Pastor-Soler N, Marciszyn A, Wen X, Gomez H, Humphries WH 4th, et al. 2017; Insulin-like growth factor binding protein 7 and tissue inhibitor of metalloproteinases-2: differential expression and secretion in human kidney tubule cells. Am J Physiol Renal Physiol. 312:F284–96. DOI: 10.1152/ajprenal.00271.2016. PMID: 28003188. PMCID: PMC5336590.8. Ortega LM, Heung M. 2018; The use of cell cycle arrest biomarkers in the early detection of acute kidney injury. Is this the new renal troponin? Nefrologia (Engl Ed). 38:361–7. DOI: 10.1016/j.nefro.2017.11.013. PMID: 29627229.9. Fan W, Ankawi G, Zhang J, Digvijay K, Giavarina D, Yin Y, et al. 2019; Current understanding and future directions in the application of TIMP-2 and IGFBP7 in AKI clinical practice. Clin Chem Lab Med. 57:567–76. DOI: 10.1515/cclm-2018-0776. PMID: 30179848.10. Edwards JK. 2015; How precise is NephroCheck®? Nat Rev Nephrol. 11:127. DOI: 10.1038/nrneph.2015.7. PMID: 25643663.11. Loupy A, Haas M, Roufosse C, Naesens M, Adam B, Afrouzian M, et al. 2020; The Banff 2019 Kidney Meeting Report (I): updates on and clarification of criteria for T cell- and antibody-mediated rejection. Am J Transplant. 20:2318–31. DOI: 10.1111/ajt.15898. PMID: 32463180. PMCID: PMC7496245.12. Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. 2009; KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 9 Suppl 3:S1–155. DOI: 10.1111/j.1600-6143.2009.02834.x. PMID: 19845597.13. Glassock RJ. 2015; Con: kidney biopsy: an irreplaceable tool for patient management in nephrology. Nephrol Dial Transplant. 30:528–31. DOI: 10.1093/ndt/gfv044. PMID: 25801636.14. Price PM, Safirstein RL, Megyesi J. 2009; The cell cycle and acute kidney injury. Kidney Int. 76:604–13. DOI: 10.1038/ki.2009.224. PMID: 19536080. PMCID: PMC2782725.15. Pajenda S, Ilhan-Mutlu A, Preusser M, Roka S, Druml W, Wagner L. 2015; NephroCheck data compared to serum creatinine in various clinical settings. BMC Nephrol. 16:206. DOI: 10.1186/s12882-015-0203-5. PMID: 26651477. PMCID: PMC4674950.16. Wang W, Saad A, Herrmann SM, Eirin Massat A, McKusick MA, Misra S, et al. 2016; Changes in inflammatory biomarkers after renal revascularization in atherosclerotic renal artery stenosis. Nephrol Dial Transplant. 31:1437–43. DOI: 10.1093/ndt/gfv448. PMID: 26908767. PMCID: PMC5009289.17. Di Leo L, Nalesso F, Garzotto F, Xie Y, Yang B, Virzì GM, et al. 2018; Predicting acute kidney injury in intensive care unit patients: the role of tissue inhibitor of metalloproteinases-2 and insulin-like growth factor-binding protein-7 biomarkers. Blood Purif. 45:270–7. DOI: 10.1159/000485591. PMID: 29478052.18. Bell M, Larsson A, Venge P, Bellomo R, Mårtensson J. 2015; Assessment of cell-cycle arrest biomarkers to predict early and delayed acute kidney injury. Dis Markers. 2015:158658. DOI: 10.1155/2015/158658. PMID: 25866432. PMCID: PMC4381987.19. El Minshawy O, Khedr MH, Youssuf AM, Abo Elela M, Kamel FM, Keryakos HK. 2021; Value of the cell cycle arrest biomarkers in the diagnosis of pregnancy-related acute kidney injury. Biosci Rep. 41:BSR20200962. DOI: 10.1042/BSR20200962. PMID: 33295613. PMCID: PMC7786331.20. Pianta TJ, Peake PW, Pickering JW, Kelleher M, Buckley NA, Endre ZH. 2015; Evaluation of biomarkers of cell cycle arrest and inflammation in prediction of dialysis or recovery after kidney transplantation. Transpl Int. 28:1392–404. DOI: 10.1111/tri.12636. PMID: 26174580.21. Yang J, Lim SY, Kim MG, Jung CW, Cho WY, Jo SK. 2017; Urinary tissue inhibitor of metalloproteinase and insulin-like growth factor-7 as early biomarkers of delayed graft function after kidney transplantation. Transplant Proc. 49:2050–4. DOI: 10.1016/j.transproceed.2017.09.023. PMID: 29149959.22. Lameire N, Vanmassenhove J, Van Biesen W, Vanholder R. 2016; The cell cycle biomarkers: promising research, but do not oversell them. Clin Kidney J. 9:353–8. DOI: 10.1093/ckj/sfw033. PMID: 27274818. PMCID: PMC4886923.23. Wever PC, Aten J, Rentenaar RJ, Hack CE, Koopman G, Weening JJ, et al. 1998; Apoptotic tubular cell death during acute renal allograft rejection. Clin Nephrol. 49:28–34.24. Hoste EA, McCullough PA, Kashani K, Chawla LS, Joannidis M, Shaw AD, et al. 2014; Derivation and validation of cutoffs for clinical use of cell cycle arrest biomarkers. Nephrol Dial Transplant. 29:2054–61. DOI: 10.1093/ndt/gfu292. PMID: 25237065. PMCID: PMC4209880.25. Tinel C, Devresse A, Vermorel A, Sauvaget V, Marx D, Avettand-Fenoel V, et al. 2020; Development and validation of an optimized integrative model using urinary chemokines for noninvasive diagnosis of acute allograft rejection. Am J Transplant. 20:3462–76. DOI: 10.1111/ajt.15959. PMID: 32342614.26. Muthukumar T, Dadhania D, Ding R, Snopkowski C, Naqvi R, Lee JB, et al. 2005; Messenger RNA for FOXP3 in the urine of renal-allograft recipients. N Engl J Med. 353:2342–51. DOI: 10.1056/NEJMoa051907. PMID: 16319383.27. Li B, Hartono C, Ding R, Sharma VK, Ramaswamy R, Qian B, et al. 2001; Noninvasive diagnosis of renal-allograft rejection by measurement of messenger RNA for perforin and granzyme B in urine. N Engl J Med. 344:947–54. DOI: 10.1056/NEJM200103293441301. PMID: 11274620.28. Ramirez-Sandoval JC, Herrington W, Morales-Buenrostro LE. 2015; Neutrophil gelatinase-associated lipocalin in kidney transplantation: a review. Transplant Rev (Orlando). 29:139–44. DOI: 10.1016/j.trre.2015.04.004. PMID: 26071983.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Histopathological findings of indication kidney allograft biopsy: 16-year experience from a tertiary care center in Korea
- The Impact of Acute Rejection on Long-Term Graft Outcome in Renal Allograft Recipient
- A Retrospective Analysis on Reasons for Allograft Nephrectomy after Kidney Transplantation
- The Outcome of Renal Transplantation Using Exchange Donor Program
- Outcomes of Kidney Transplantation from Spousal Donors: Comparison with Kidney Transplantation from Living-related Donors