J Rheum Dis.
2024 Jan;31(1):3-14. 10.4078/jrd.2023.0042.
Radiologic approach and progressive exploration of connective tissue disease-related interstitial lung disease: meeting the curiosity of rheumatologists
- Affiliations
-
- 1Division of Rheumatology, Department of Internal Medicine, Soonchunhyang University Seoul Hospital, Soonchunhyang University College of Medicine, Seoul, Korea
- 2Department of Radiology, Soonchunhyang University Seoul Hospital, Soonchunhyang University College of Medicine, Seoul, Korea
- 3Division of Rheumatology, Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2549631
- DOI: http://doi.org/10.4078/jrd.2023.0042
Abstract
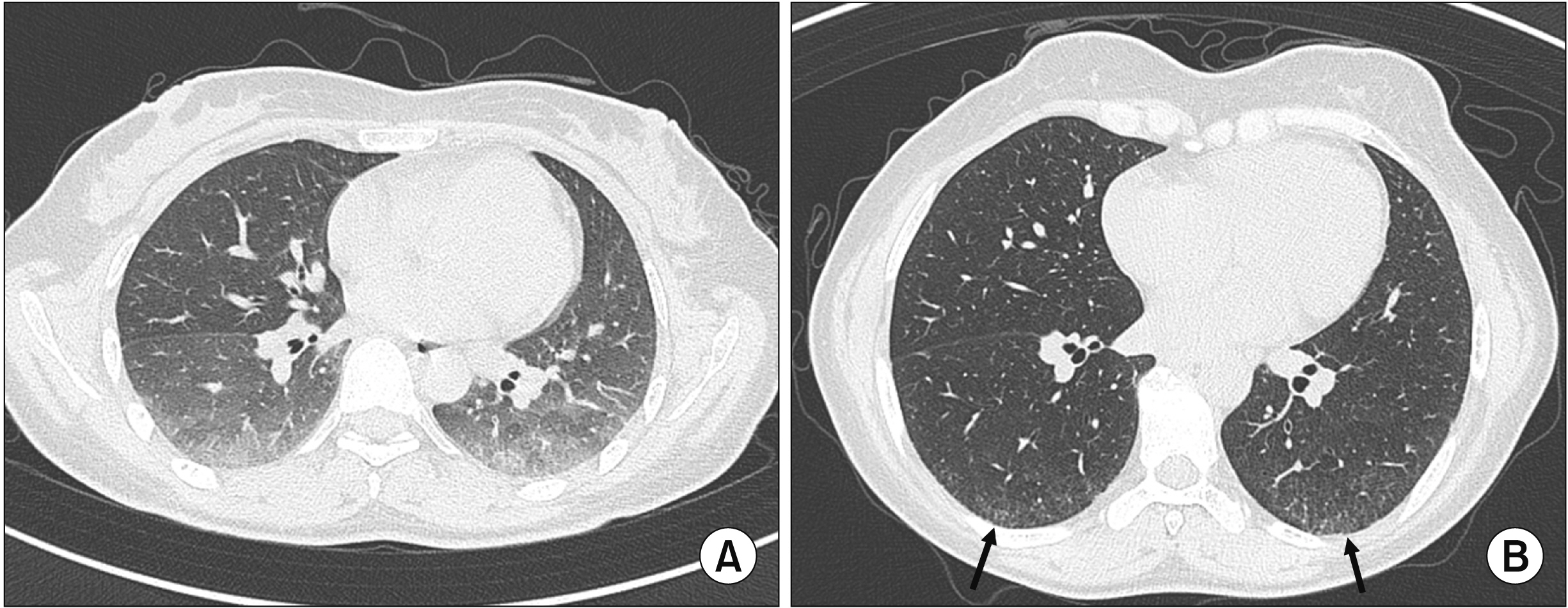
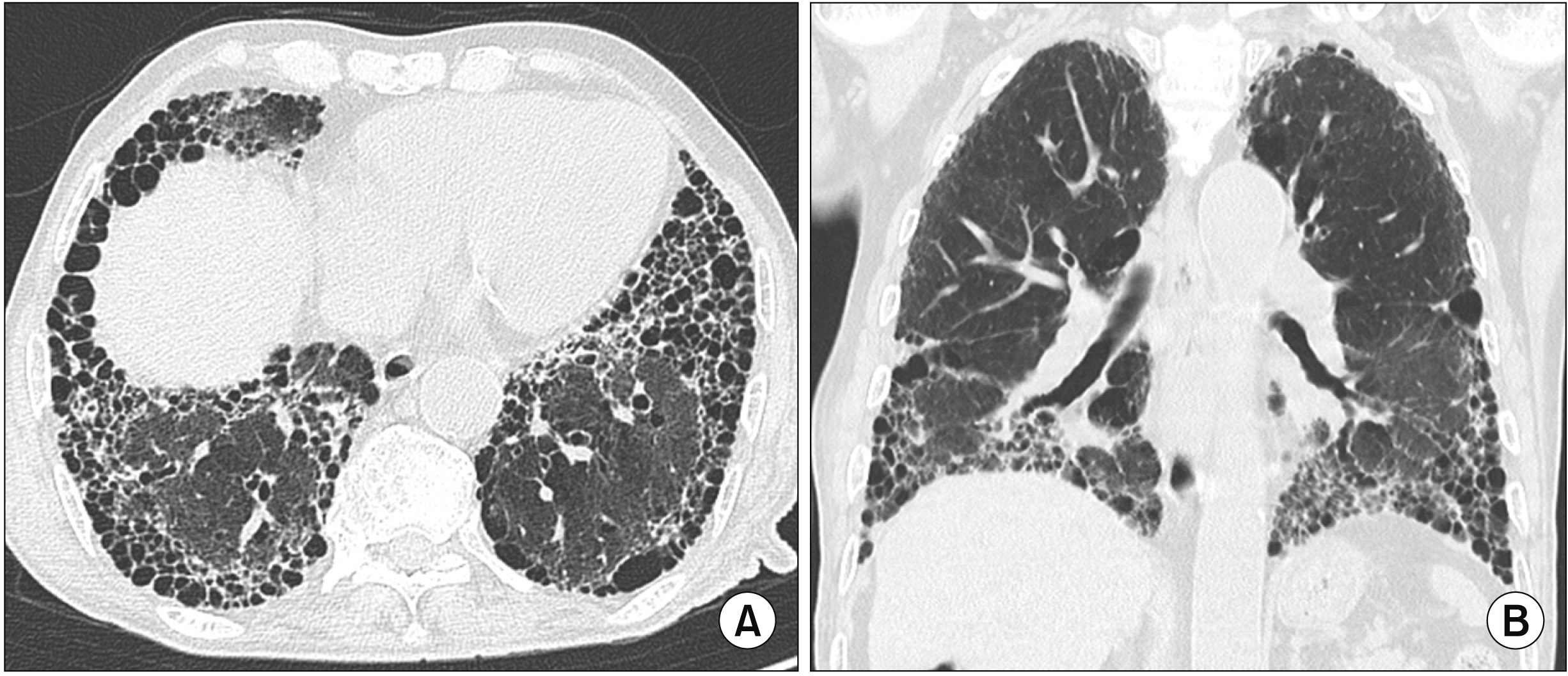
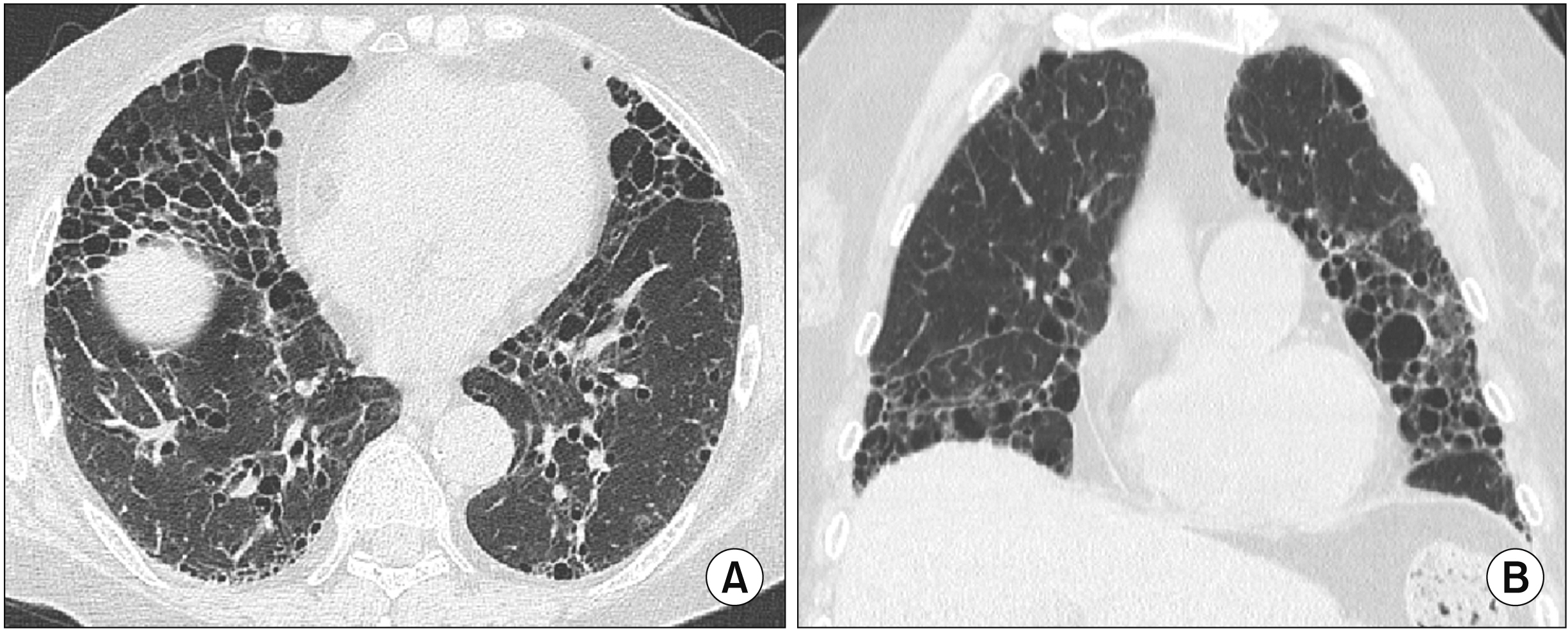
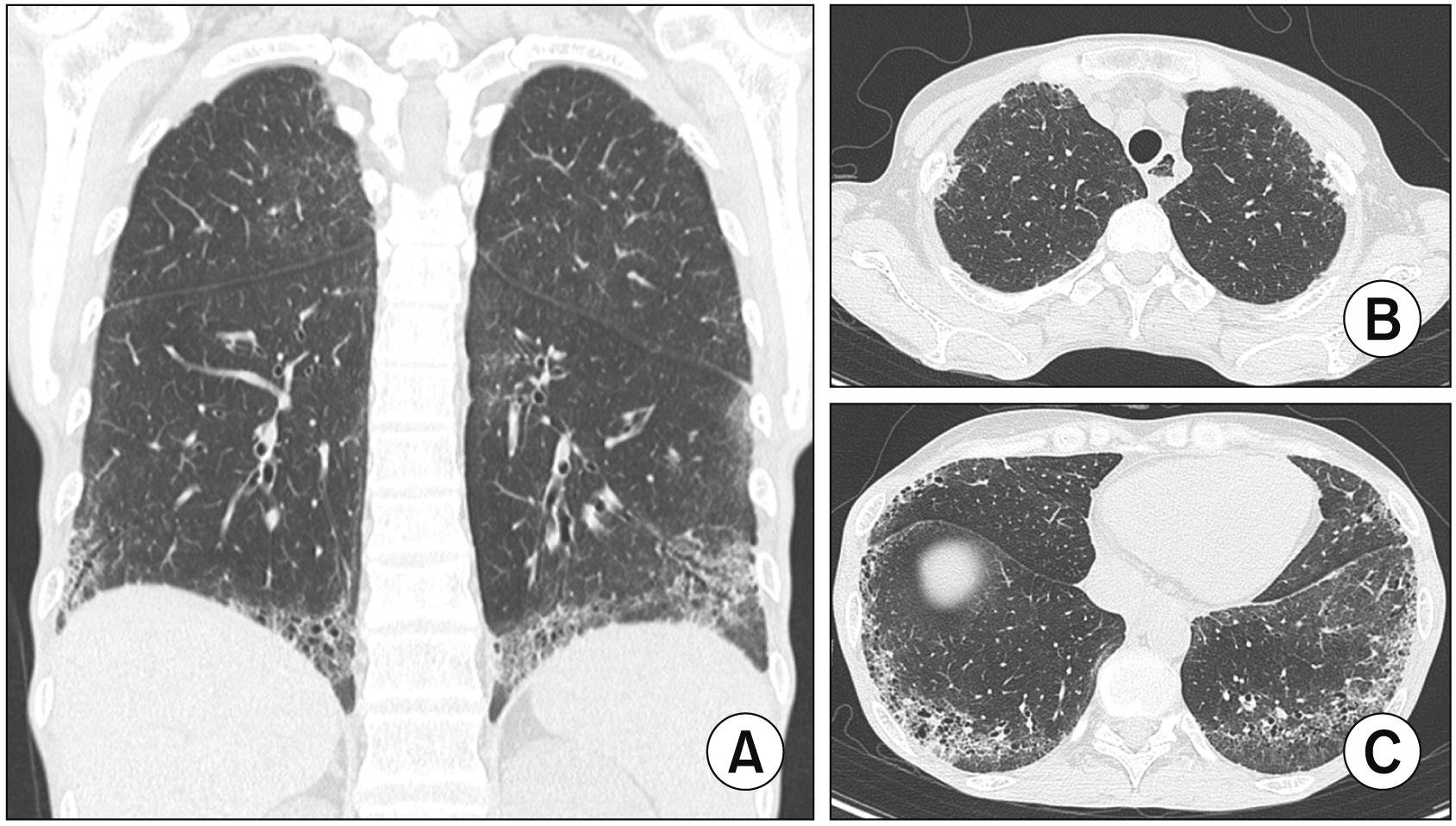
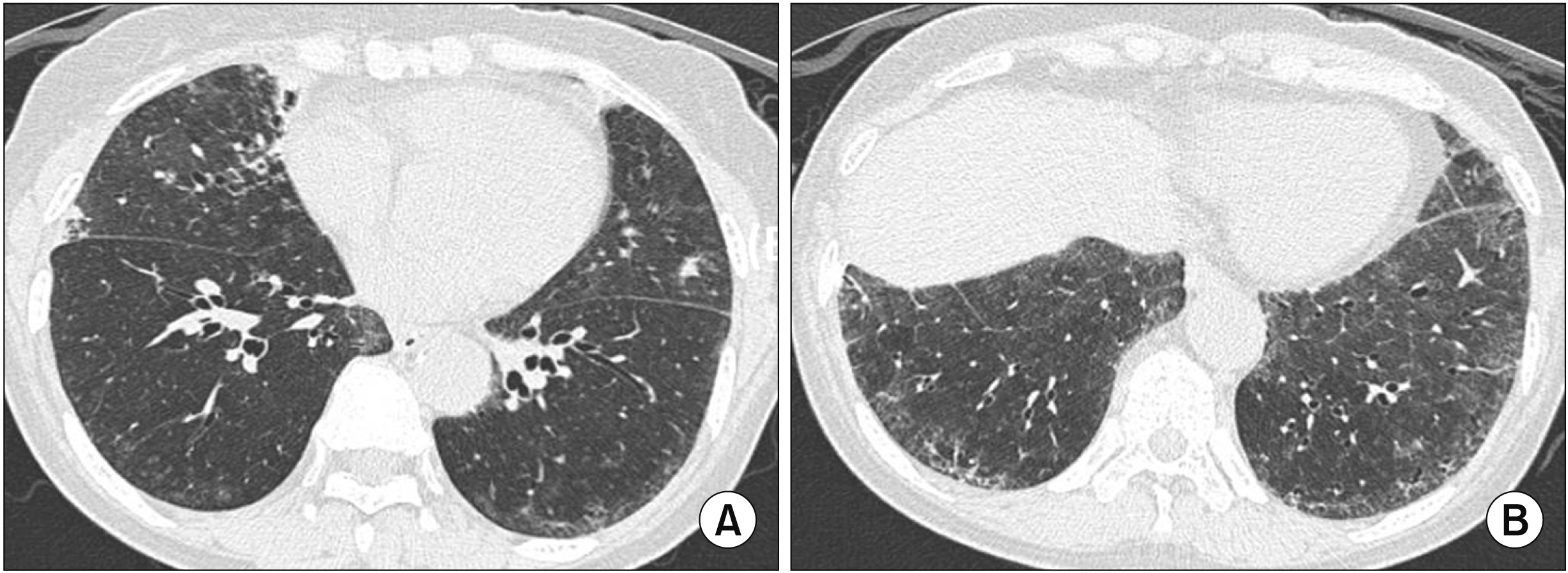
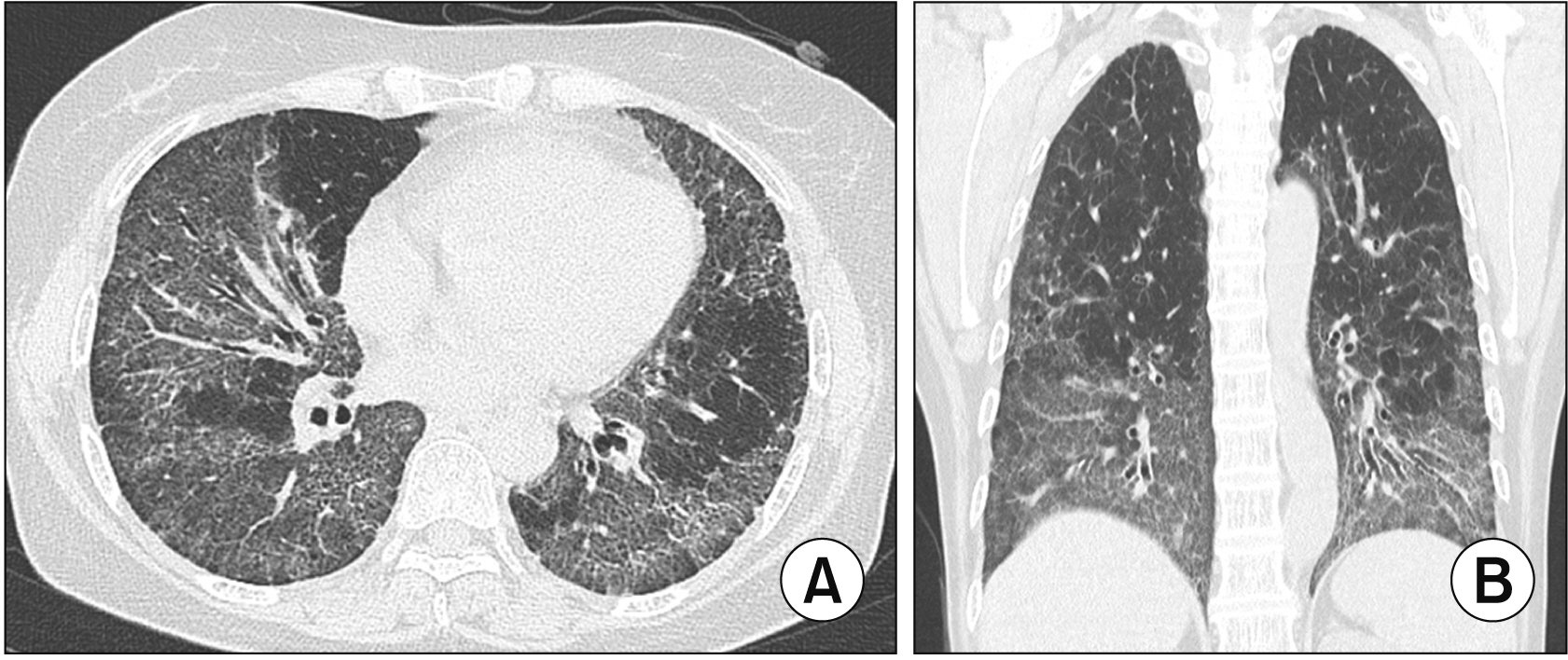
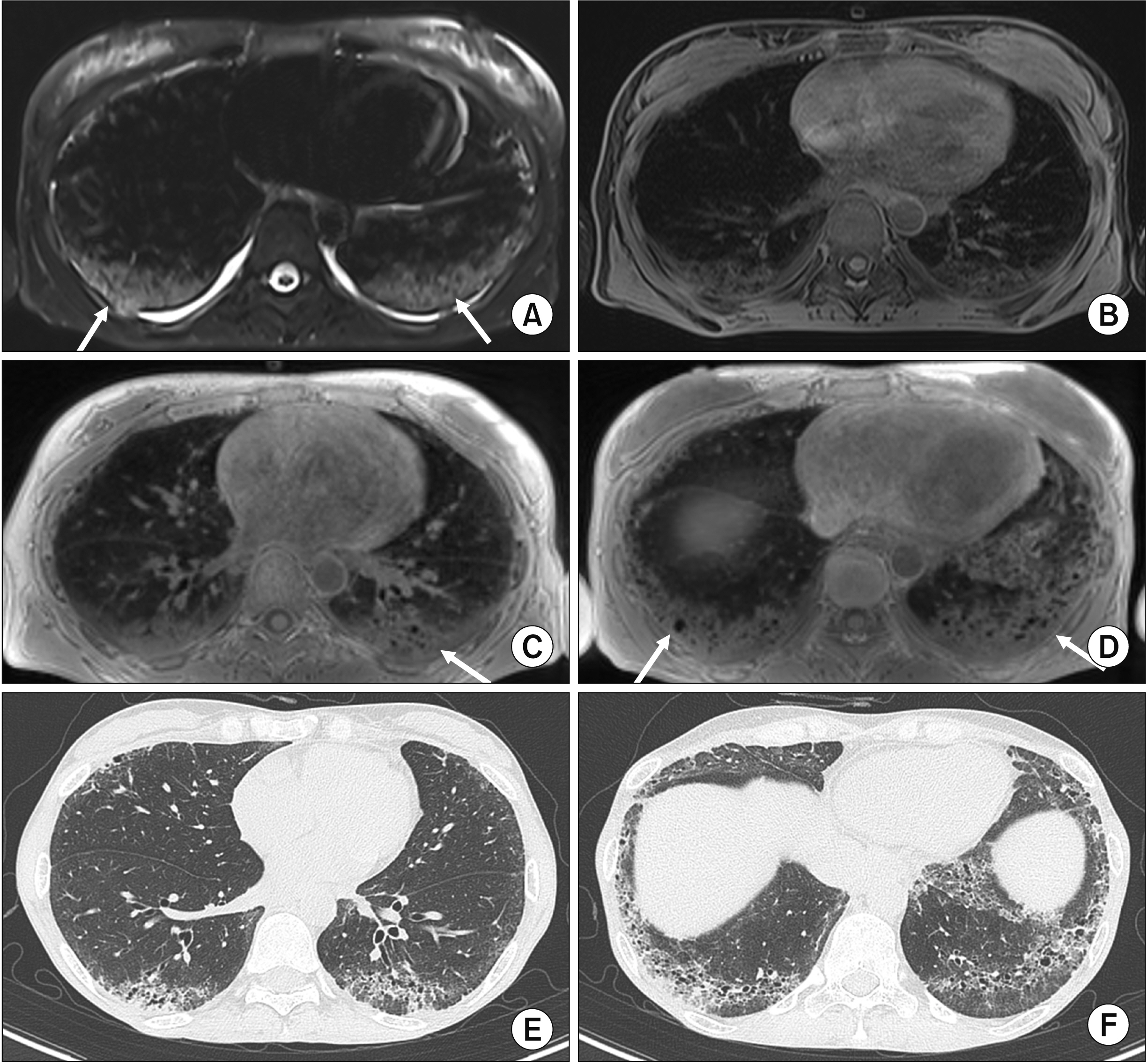
- Interstitial lung disease (ILD) is often observed in connective tissue diseases (CTDs), frequently in rheumatoid arthritis, systemic sclerosis, primary Sjögren’s syndrome, and inflammatory myositis. Early detection of ILDs secondary to rheumatic diseases is important as timely initiation of proper management affects the prognosis. Among many imaging modalities, high-resuloution computed tomography (HRCT) serves the gold standard for finding early lung inflammatory and fibrotic changes as well as monitoring afterwards because of its superior spatial resolution. Additionally, lung ultrasound (LUS) and magnetic resonance imaging (MRI) are the rising free-radiation imaging tools that can get images of lungs of CTD-ILD. In this review article, we present the subtypes of ILD images found in each CTD acquired by HRCT as well as some images taken by LUS and MRI with comparative HRCT scans. It is expected that this discussion would be helpful in discussing recent advances in imaging modalities for CTDILD and raising critical points for diagnosis and tracing of the images from the perspective of rheumatologists.
Figure
Reference
-
1. Koo SM, Kim SY, Choi SM, Lee HK. Korean Interstitial Lung Diseases Study Group. 2019; Korean guidelines for diagnosis and management of interstitial lung diseases: part 5. Connective tissue disease associated interstitial lung disease. Tuberc Respir Dis (Seoul). 82:285–97. DOI: 10.4046/trd.2019.0009. PMID: 31172701. PMCID: PMC6778739.
Article2. Fischer A, du Bois R. 2012; Interstitial lung disease in connective tissue disorders. Lancet. 380:689–98. Erratum in: Lancet 2012;380:1148. DOI: 10.1016/S0140-6736(12)61079-4. PMID: 22901890.
Article3. Castelino FV, Goldberg H, Dellaripa PF. 2011; The impact of rheumatological evaluation in the management of patients with interstitial lung disease. Rheumatology (Oxford). 50:489–93. DOI: 10.1093/rheumatology/keq233. PMID: 20685802.
Article4. Shao T, Shi X, Yang S, Zhang W, Li X, Shu J, et al. 2021; Interstitial lung disease in connective tissue disease: a common lesion with heterogeneous mechanisms and treatment considerations. Front Immunol. 12:684699. DOI: 10.3389/fimmu.2021.684699. PMID: 34163483. PMCID: PMC8215654. PMID: 0840a79cf6c6447780393d8928e7f578.
Article5. Hansell DM. 2001; Computed tomography of diffuse lung disease: functional correlates. Eur Radiol. 11:1666–80. DOI: 10.1007/s003300100880. PMID: 11511888.6. Sauleda J, Núñez B, Sala E, Soriano JB. 2018; Idiopathic pulmonary fibrosis: epidemiology, natural history, phenotypes. Med Sci (Basel). 6:110. DOI: 10.3390/medsci6040110. PMID: 30501130. PMCID: PMC6313500. PMID: fbd7a9ebbecc4762b2c0a396ae99487d.7. Song JW. 2014; Interstitial lung disease in connective tissue disease. J Rheum Dis. 21:282–8. DOI: 10.4078/jrd.2014.21.6.282.
Article8. Vij R, Strek ME. 2013; Diagnosis and treatment of connective tissue disease-associated interstitial lung disease. Chest. 143:814–24. DOI: 10.1378/chest.12-0741. PMID: 23460159. PMCID: PMC3590889.
Article9. Duman D. 2022; Progressive pulmonary fibrosis (PPF). Tuberk Toraks. 70:375–81. DOI: 10.5578/tt.20229609. PMID: 36537095.
Article10. Walsh SLF, Devaraj A, Enghelmayer JI, Kishi K, Silva RS, Patel N, et al. 2018; Role of imaging in progressive-fibrosing interstitial lung diseases. Eur Respir Rev. 27:180073. DOI: 10.1183/16000617.0073-2018. PMID: 30578332. PMCID: PMC9488692. PMID: 349d2b2dcdaf42fb840f2cb8db66ead4.
Article11. Cottin V, Hirani NA, Hotchkin DL, Nambiar AM, Ogura T, Otaola M, et al. 2018; Presentation, diagnosis and clinical course of the spectrum of progressive-fibrosing interstitial lung diseases. Eur Respir Rev. 27:180076. DOI: 10.1183/16000617.0076-2018. PMID: 30578335. PMCID: PMC9489068. PMID: 7a3b048e30e34a06a74e12a5a72e396b.
Article12. Torres PPTES, Rabahi MF, Moreira MADC, Escuissato DL, Meirelles GSP, Marchiori E. 2021; Importance of chest HRCT in the diagnostic evaluation of fibrosing interstitial lung diseases. J Bras Pneumol. 47:e20200096. DOI: 10.36416/1806-3756/e20200096. PMID: 34076172. PMCID: PMC8332714. PMID: 7cbd1fa17f4f4ebcb304e1e9608d924b.13. Raghu G, Remy-Jardin M, Richeldi L, Thomson CC, Inoue Y, Johkoh T, et al. 2022; Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 205:e18–47. DOI: 10.1164/rccm.202202-0399ST. PMID: 35486072. PMCID: PMC9851481.14. Pugashetti JV, Adegunsoye A, Wu Z, Lee CT, Srikrishnan A, Ghodrati S, et al. 2023; Validation of proposed criteria for progressive pulmonary fibrosis. Am J Respir Crit Care Med. 207:69–76. DOI: 10.1164/rccm.202201-0124OC. PMID: 35943866.
Article15. Emilsson ÖI, Dessle A, Johansson H, Adeli S, Malinovschi A, Eloranta ML, et al. 2022; Different chest HRCT scan protocols change the extent of ground glass opacities. BMC Pulm Med. 22:430. DOI: 10.1186/s12890-022-02212-7. PMID: 36404311. PMCID: PMC9677886. PMID: b5406b35c5da4bddb3a4aaad36119649.
Article16. Tanaka N, Kim JS, Newell JD, Brown KK, Cool CD, Meehan R, et al. 2004; Rheumatoid arthritis-related lung diseases: CT findings. Radiology. 232:81–91. DOI: 10.1148/radiol.2321030174. PMID: 15166329.
Article17. Lynch DA, Sverzellati N, Travis WD, Brown KK, Colby TV, Galvin JR, et al. 2018; Diagnostic criteria for idiopathic pulmonary fibrosis: a Fleischner Society White Paper. Lancet Respir Med. 6:138–53. DOI: 10.1016/S2213-2600(17)30433-2. PMID: 29154106.
Article18. Balbir-Gurman A, Guralnik L, Yigla M, Braun-Moscovici Y, Hardak E. 2018; Imaging aspects of interstitial lung disease in patients with rheumatoid arthritis: literature review. Autoimmun Rev. 17:87–93. DOI: 10.1016/j.autrev.2017.09.013. PMID: 29196242.
Article19. Smith M, Dalurzo M, Panse P, Parish J, Leslie K. 2013; Usual interstitial pneumonia-pattern fibrosis in surgical lung biopsies. Clinical, radiological and histopathological clues to aetiology. J Clin Pathol. 66:896–903. DOI: 10.1136/jclinpath-2013-201442. PMID: 23703852. PMCID: PMC3786616.
Article20. Hoffmann-Vold AM, Fretheim H, Halse AK, Seip M, Bitter H, Wallenius M, et al. 2019; Tracking impact of interstitial lung disease in systemic sclerosis in a complete nationwide cohort. Am J Respir Crit Care Med. 200:1258–66. DOI: 10.1164/rccm.201903-0486OC. PMID: 31310156.
Article21. Khanna SA, Nance JW, Suliman SA. 2022; Detection and monitoring of interstitial lung disease in patients with systemic sclerosis. Curr Rheumatol Rep. 24:166–73. Erratum in: Curr Rheumatol Rep 2022;24:321. DOI: 10.1007/s11926-022-01067-5. PMID: 35499699. PMCID: PMC9399070.
Article22. Luppi F, Sebastiani M, Silva M, Sverzellati N, Cavazza A, Salvarani C, et al. 2020; Interstitial lung disease in Sjögren's syndrome: a clinical review. Clin Exp Rheumatol. 38 Suppl 126(4):291–300.23. Lohrmann C, Uhl M, Warnatz K, Ghanem N, Kotter E, Schaefer O, et al. 2004; High-resolution CT imaging of the lung for patients with primary Sjogren's syndrome. Eur J Radiol. 52:137–43. DOI: 10.1016/j.ejrad.2004.01.006. PMID: 15489070.24. Nakanishi M, Fukuoka J, Tanaka T, Demura Y, Umeda Y, Ameshima S, et al. 2011; Small airway disease associated with Sjögren's syndrome: clinico-pathological correlations. Respir Med. 105:1931–8. DOI: 10.1016/j.rmed.2011.08.009. PMID: 21903371.25. Soto-Cardenas MJ, Perez-De-Lis M, Bove A, Navarro C, Brito-Zeron P, Diaz-Lagares C, et al. 2010; Bronchiectasis in primary Sjögren's syndrome: prevalence and clinical significance. Clin Exp Rheumatol. 28:647–53. PMID: 20883638.26. Tanizawa K, Handa T, Nakashima R, Kubo T, Hosono Y, Aihara K, et al. 2013; The prognostic value of HRCT in myositis-associated interstitial lung disease. Respir Med. 107:745–52. DOI: 10.1016/j.rmed.2013.01.014. PMID: 23485097.
Article27. Gutsche M, Rosen GD, Swigris JJ. 2012; Connective tissue disease-associated interstitial lung disease: a review. Curr Respir Care Rep. 1:224–32. DOI: 10.1007/s13665-012-0028-7. PMID: 23125954. PMCID: PMC3486427.
Article28. Marie I, Hachulla E, Chérin P, Dominique S, Hatron PY, Hellot MF, et al. 2002; Interstitial lung disease in polymyositis and dermatomyositis. Arthritis Rheum. 47:614–22. DOI: 10.1002/art.10794. PMID: 12522835.
Article29. Vizioli L, Ciccarese F, Forti P, Chiesa AM, Giovagnoli M, Mughetti M, et al. 2017; Integrated use of lung ultrasound and chest X-ray in the detection of interstitial lung disease. Respiration. 93:15–22. DOI: 10.1159/000452225. PMID: 27880957.
Article30. European Society of Radiology (ESR). 2021; The role of lung ultrasound in COVID-19 disease. Insights Imaging. 12:81. DOI: 10.1186/s13244-021-01013-6. PMID: 34146161. PMCID: PMC8214066. PMID: 31ae9b35463a40a99ef2108b289fb9c2.31. Volpicelli G. 2013; Lung sonography. J Ultrasound Med. 32:165–71. DOI: 10.7863/jum.2013.32.1.165. PMID: 23269722.
Article32. Tinti MG, Rea G, Mirijello A, Frongillo E, Sperandeo M. 2017; Is there any role for thoracic ultrasound for interstitial lung disease underlying rheumatologic conditions? Comment. Intern Emerg Med. 12:903–4. DOI: 10.1007/s11739-017-1663-3. PMID: 28401427.
Article33. Moazedi-Fuerst FC, Kielhauser SM, Scheidl S, Tripolt NJ, Lutfi A, Yazdani-Biuki B, et al. 2014; Ultrasound screening for interstitial lung disease in rheumatoid arthritis. Clin Exp Rheumatol. 32:199–203. PMID: 24642277.34. Marini TJ, Rubens DJ, Zhao YT, Weis J, O'Connor TP, Novak WH, et al. 2021; Lung ultrasound: the essentials. Radiol Cardiothorac Imaging. 3:e200564. DOI: 10.1148/ryct.2021200564. PMID: 33969313. PMCID: PMC8098095.
Article35. Huang Y, Liu T, Huang S, Qiu L, Luo F, Yin G, et al. 2023; Screening value of lung ultrasound in connective tissue disease related interstitial lung disease. Heart Lung. 57:110–6. DOI: 10.1016/j.hrtlng.2022.09.011. PMID: 36182861.
Article36. Sperandeo M, Varriale A, Sperandeo G, Filabozzi P, Piattelli ML, Carnevale V, et al. 2009; Transthoracic ultrasound in the evaluation of pulmonary fibrosis: our experience. Ultrasound Med Biol. 35:723–9. DOI: 10.1016/j.ultrasmedbio.2008.10.009. PMID: 19111972.
Article37. Gargani L, Doveri M, D'Errico L, Frassi F, Bazzichi ML, Delle Sedie A, et al. 2009; Ultrasound lung comets in systemic sclerosis: a chest sonography hallmark of pulmonary interstitial fibrosis. Rheumatology (Oxford). 48:1382–7. DOI: 10.1093/rheumatology/kep263. PMID: 19717549.
Article38. Picano E, Matucci-Cerinic M. 2011; Unnecessary radiation exposure from medical imaging in the rheumatology patient. Rheumatology (Oxford). 50:1537–9. DOI: 10.1093/rheumatology/keq412. PMID: 21183451.
Article39. Barreto MM, Rafful PP, Rodrigues RS, Zanetti G, Hochhegger B, Souza AS Jr, et al. 2013; Correlation between computed tomographic and magnetic resonance imaging findings of parenchymal lung diseases. Eur J Radiol. 82:e492–501. DOI: 10.1016/j.ejrad.2013.04.037. PMID: 23763860.
Article40. Tiddens HA, Stick SM, Wild JM, Ciet P, Parker GJ, Koch A, et al. 2015; Respiratory tract exacerbations revisited: ventilation, inflammation, perfusion, and structure (VIPS) monitoring to redefine treatment. Pediatr Pulmonol. 50 Suppl 40:S57–65. DOI: 10.1002/ppul.23266.
Article41. Shioya S, Tsuji C, Kurita D, Katoh H, Tsuda M, Haida M, et al. 1997; Early damage to lung tissue after irradiation detected by the magnetic resonance T2 relaxation time. Radiat Res. 148:359–64. DOI: 10.2307/3579521. PMID: 9339952.
Article42. Lutterbey G, Grohé C, Gieseke J, von Falkenhausen M, Morakkabati N, Wattjes MP, et al. 2007; Initial experience with lung-MRI at 3.0T: Comparison with CT and clinical data in the evaluation of interstitial lung disease activity. Eur J Radiol. 61:256–61. DOI: 10.1016/j.ejrad.2006.09.005. PMID: 17034975.
Article43. Hekimoğlu K, Sancak T, Tor M, Beşir H, Kalaycioğlu B, Gündoğdu S. 2010; Fast MRI evaluation of pulmonary progressive massive fibrosis with VIBE and HASTE sequences: comparison with CT. Diagn Interv Radiol. 16:30–7. DOI: 10.4261/1305-3825.DIR.2313-08.2. PMID: 20027548.44. Pinal-Fernandez I, Pineda-Sanchez V, Pallisa-Nuñez E, Simeon-Aznar CP, Selva-O'Callaghan A, Fonollosa-Pla V, et al. 2016; Fast 1.5 T chest MRI for the assessment of interstitial lung disease extent secondary to systemic sclerosis. Clin Rheumatol. 35:2339–45. DOI: 10.1007/s10067-016-3267-0. PMID: 27107755.
Article45. Hochhegger B, de Souza VV, Marchiori E, Irion KL, Souza AS Jr, Elias Junior J, et al. 2015; Chest magnetic resonance imaging: a protocol suggestion. Radiol Bras. 48:373–80. DOI: 10.1590/0100-3984.2014.0017. PMID: 26811555. PMCID: PMC4725399.
Article46. Rajaram S, Swift AJ, Capener D, Telfer A, Davies C, Hill C, et al. 2012; Lung morphology assessment with balanced steady-state free precession MR imaging compared with CT. Radiology. 263:569–77. DOI: 10.1148/radiol.12110990. PMID: 22396606.
Article47. Lonzetti L, Zanon M, Pacini GS, Altmayer S, Martins de Oliveira D, Rubin AS, et al. 2019; Magnetic resonance imaging of interstitial lung diseases: a state-of-the-art review. Respir Med. 155:79–85. DOI: 10.1016/j.rmed.2019.07.006. PMID: 31323528.48. Romei C, Turturici L, Tavanti L, Miedema J, Fiorini S, Marletta M, et al. 2018; The use of chest magnetic resonance imaging in interstitial lung disease: a systematic review. Eur Respir Rev. 27:180062. DOI: 10.1183/16000617.0062-2018. PMID: 30567932. PMCID: PMC9489061. PMID: a089903c02314712989d16709ebc8403.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Connective Tissue Disease-Associated Interstitial Lung Disease
- Interstitial Lung Diseases: Respiratory Review of 2013
- Interstitial Lung Disease in Connective Tissue Disease
- A Radiologic Approach to Diffuse Interstitial Lung Disease
- Impact of Rheumatologic Consultations on Detecting Interstitial Lung Disease Associated with Connective Tissue Disease