Endocrinol Metab.
2023 Dec;38(6):760-769. 10.3803/EnM.2023.1774.
AM1638, a GPR40-Full Agonist, Inhibited Palmitate- Induced ROS Production and Endoplasmic Reticulum Stress, Enhancing HUVEC Viability in an NRF2-Dependent Manner
- Affiliations
-
- 1BK21 Graduate Program, Department of Biomedical Sciences, Korea University College of Medicine, Seoul, Korea
- 2Division of Endocrinology and Metabolism, Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea
- KMID: 2549269
- DOI: http://doi.org/10.3803/EnM.2023.1774
Abstract
- Background
G protein-coupled receptor 40 (GPR40) is a key molecule in diabetes and fatty liver, but its role in endothelial dysfunction remains unclear. Our objective in this study was to determine whether GPR40 agonists protect endothelial cells against palmitatemediated oxidative stress.
Methods
Human umbilical vein endothelial cells (HUVECs) were used to investigate effects of various GPR40 agonists on vascular endothelium.
Results
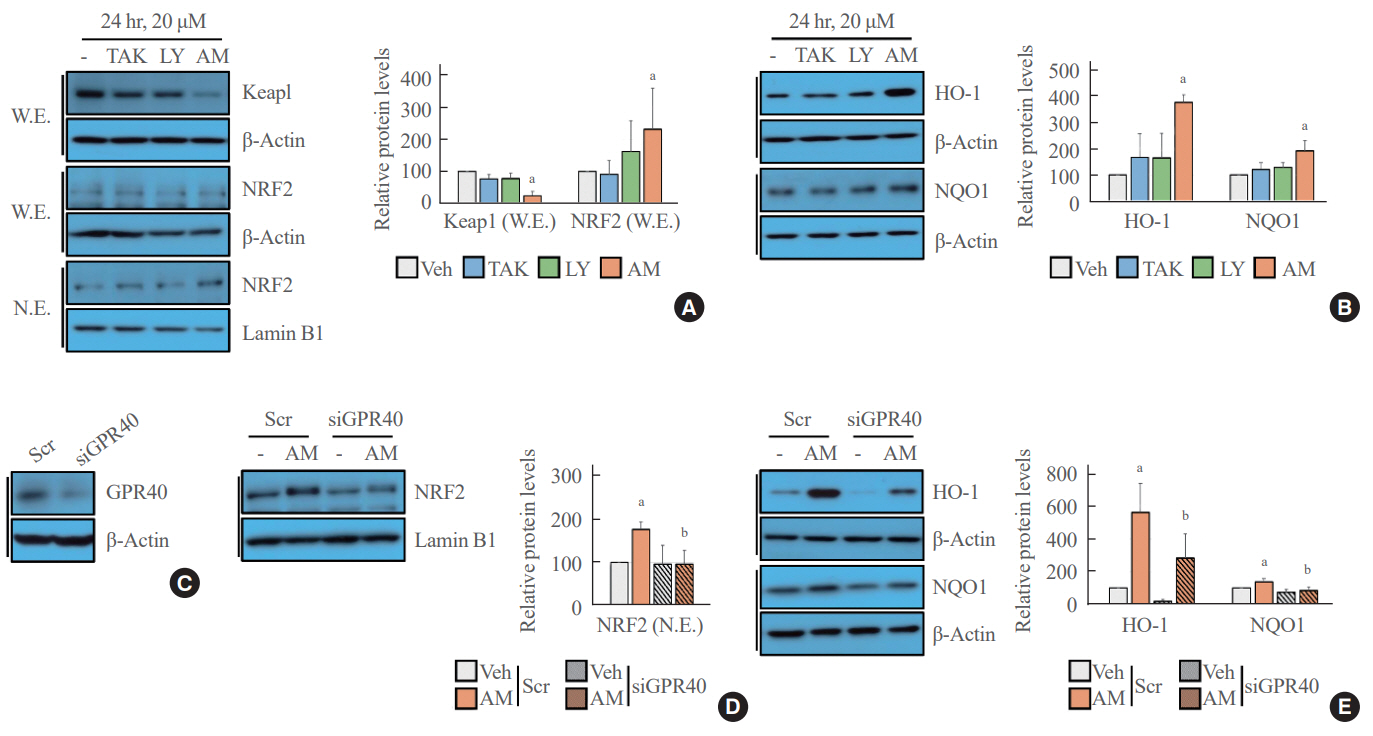
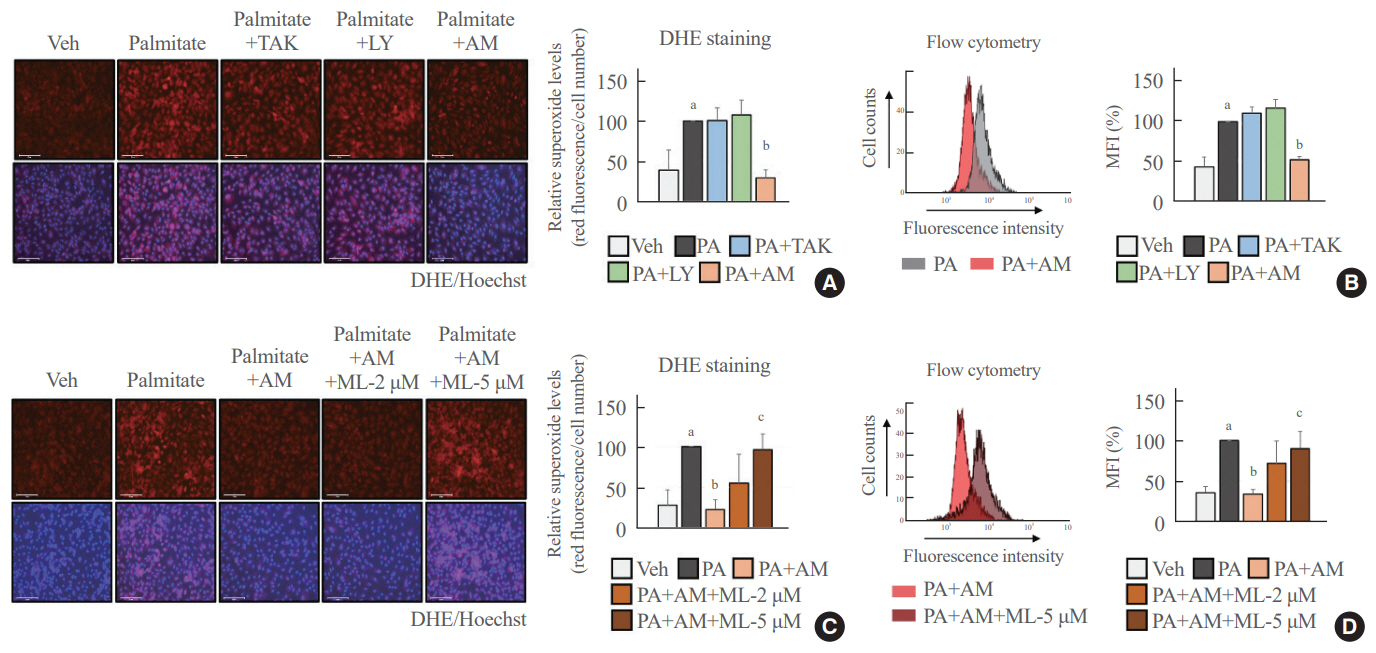
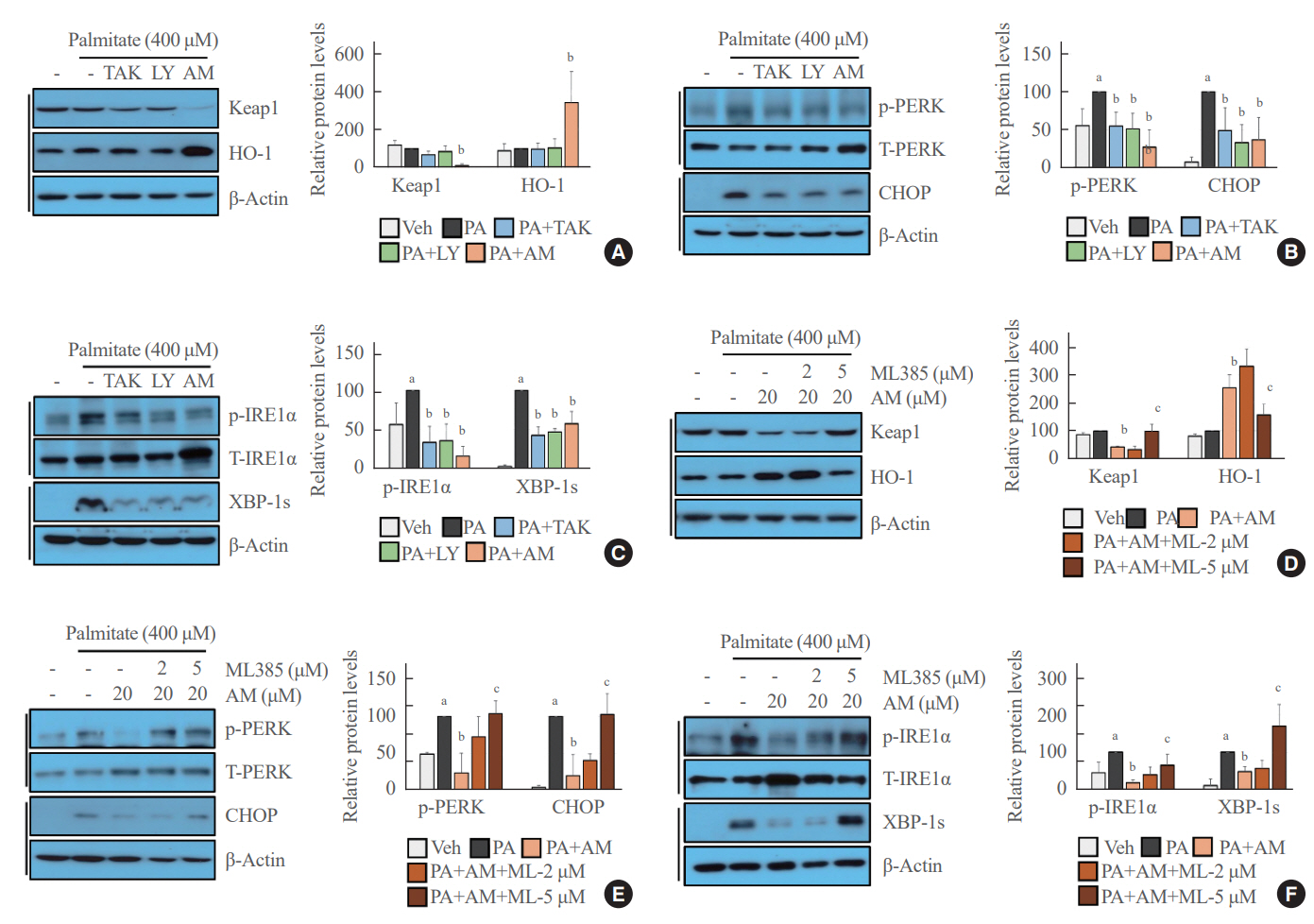
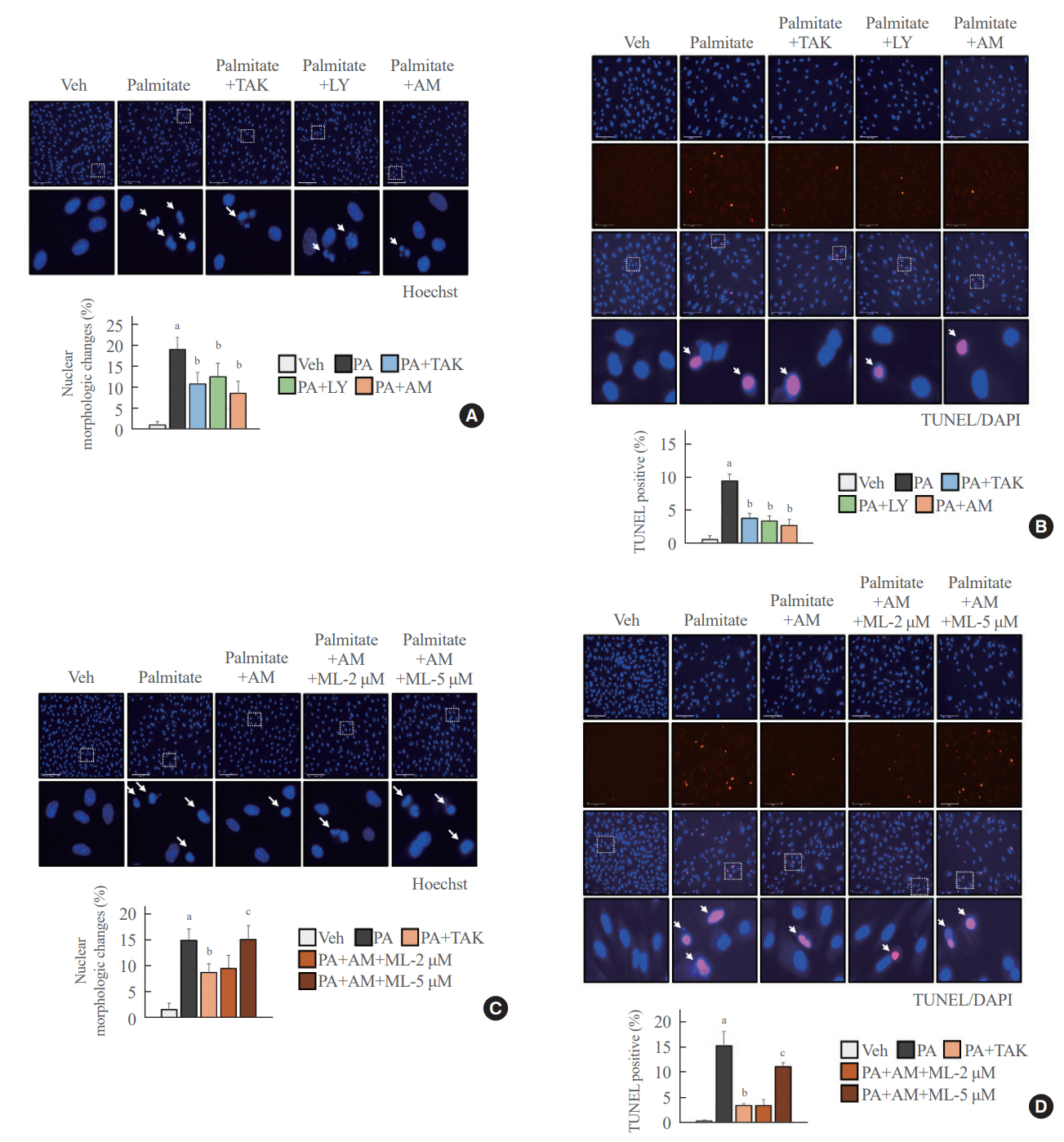
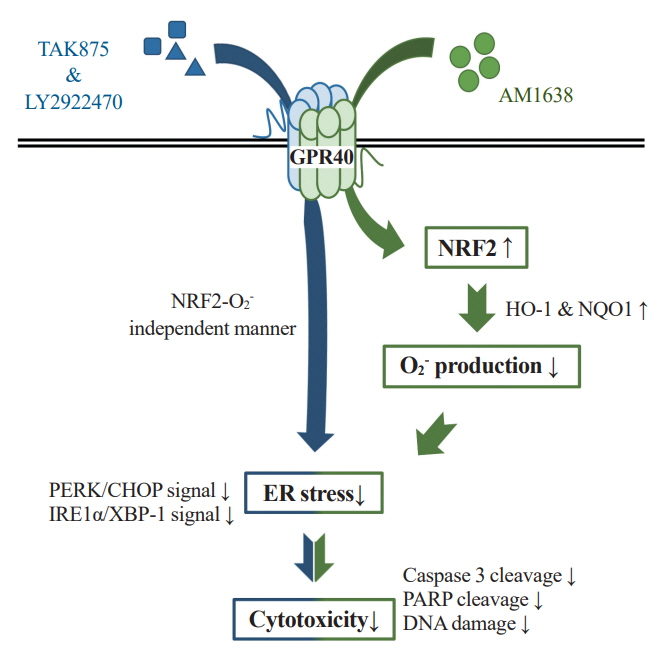
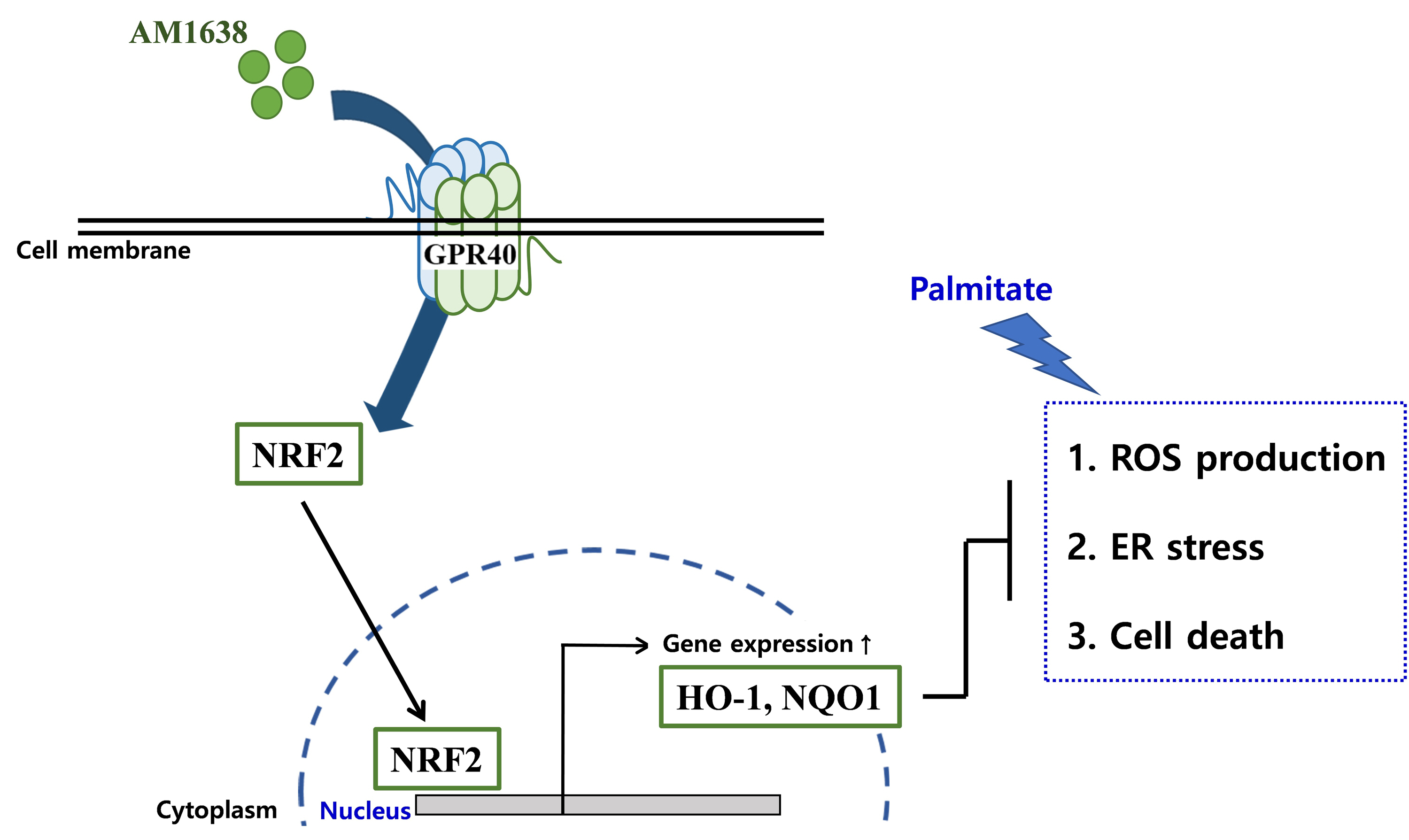
In HUVECs, AM1638, a GPR40-full agonist, enhanced nuclear factor erythroid 2–related factor 2 (NRF2) translocation to the nucleus and heme oxygenase-1 (HO-1) expression, which blocked palmitate-induced superoxide production. Those antioxidant effects were not detected after treatment with LY2922470 or TAK875, GPR40-partial agonists, suggesting that GPR40 regulates reactive oxygen species (ROS) removal in a ligand-dependent manner. We also found that palmitate-induced CCAAT/enhancer‐binding protein homologous protein expression; X-box binding protein-1 splicing, nuclear condensation, and fragmentation; and caspase-3 cleavage were all blocked in an NRF2-dependent manner after AM1638 treatment. Both LY2922470 and TAK875 also improved cell viability independent of the NRF2/ROS pathway by reducing palmitate-mediated endoplasmic reticulum stress and nuclear damage. GPR40 agonists thus have beneficial effects against palmitate in HUVECs. In particular, AM1638 reduced palmitate-induced superoxide production and cytotoxicity in an NRF2/HO-1 dependent manner.
Conclusion
GPR40 could be developed as a good therapeutic target to prevent or treat cardiovascular diseases such as atherosclerosis.
Keyword
Figure
Reference
-
1. Rajendran P, Rengarajan T, Thangavel J, Nishigaki Y, Sakthisekaran D, Sethi G, et al. The vascular endothelium and human diseases. Int J Biol Sci. 2013; 9:1057–69.
Article2. Scioli MG, Storti G, D’Amico F, Rodriguez Guzman R, Centofanti F, Doldo E, et al. Oxidative stress and new pathogenetic mechanisms in endothelial dysfunction: potential diagnostic biomarkers and therapeutic targets. J Clin Med. 2020; 9:1995.
Article3. Sorescu D, Weiss D, Lassegue B, Clempus RE, Szocs K, Sorescu GP, et al. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation. 2002; 105:1429–35.
Article4. Vendrov AE, Stevenson MD, Alahari S, Pan H, Wickline SA, Madamanchi NR, et al. Attenuated superoxide dismutase 2 activity induces atherosclerotic plaque instability during aging in hyperlipidemic mice. J Am Heart Assoc. 2017; 6:e006775.
Article5. Sawzdargo M, George SR, Nguyen T, Xu S, Kolakowski LF, O’Dowd BF. A cluster of four novel human G protein-coupled receptor genes occurring in close proximity to CD22 gene on chromosome 19q13.1. Biochem Biophys Res Commun. 1997; 239:543–7.
Article6. Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, et al. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 2003; 422:173–6.
Article7. Kebede M, Alquier T, Latour MG, Semache M, Tremblay C, Poitout V. The fatty acid receptor GPR40 plays a role in insulin secretion in vivo after high-fat feeding. Diabetes. 2008; 57:2432–7.
Article8. Nagasumi K, Esaki R, Iwachidow K, Yasuhara Y, Ogi K, Tanaka H, et al. Overexpression of GPR40 in pancreatic beta-cells augments glucose-stimulated insulin secretion and improves glucose tolerance in normal and diabetic mice. Diabetes. 2009; 58:1067–76.9. Rani L, Grewal AS, Sharma N, Singh S. Recent updates on free fatty acid receptor 1 (GPR-40) agonists for the treatment of type 2 diabetes mellitus. Mini Rev Med Chem. 2021; 21:426–70.
Article10. Verma MK, Sadasivuni MK, Yateesh AN, Neelima K, Mrudula S, Reddy M, et al. Activation of GPR40 attenuates chronic inflammation induced impact on pancreatic β-cells health and function. BMC Cell Biol. 2014; 15:24.
Article11. Jeon SM. Regulation and function of AMPK in physiology and diseases. Exp Mol Med. 2016; 48:e245.
Article12. Li M, Meng X, Xu J, Huang X, Li H, Li G, et al. GPR40 agonist ameliorates liver X receptor-induced lipid accumulation in liver by activating AMPK pathway. Sci Rep. 2016; 6:25237.
Article13. Ookawara M, Matsuda K, Watanabe M, Moritoh Y. The GPR40 full agonist SCO-267 improves liver parameters in a mouse model of nonalcoholic fatty liver disease without affecting glucose or body weight. J Pharmacol Exp Ther. 2020; 375:21–7.
Article14. Kim JW, Roh E, Choi KM, Yoo HJ, Hwang HJ, Baik SH. GPR40 agonism modulates inflammatory reactions in vascular endothelial cells. Diabetes Metab J. 2022; 46:506–11.
Article15. Hauge M, Vestmar MA, Husted AS, Ekberg JP, Wright MJ, Di Salvo J, et al. GPR40 (FFAR1): combined Gs and Gq signaling in vitro is associated with robust incretin secretagogue action ex vivo and in vivo. Mol Metab. 2014; 4:3–14.
Article16. Ho JD, Chau B, Rodgers L, Lu F, Wilbur KL, Otto KA, et al. Structural basis for GPR40 allosteric agonism and incretin stimulation. Nat Commun. 2018; 9:1645.
Article17. Rives ML, Rady B, Swanson N, Zhao S, Qi J, Arnoult E, et al. GPR40-mediated Gα12 activation by allosteric full agonists highly efficacious at potentiating glucose-stimulated insulin secretion in human islets. Mol Pharmacol. 2018; 93:581–91.
Article18. Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013; 53:401–26.
Article19. Araujo JA, Zhang M, Yin F. Heme oxygenase-1, oxidation, inflammation, and atherosclerosis. Front Pharmacol. 2012; 3:119.
Article20. Fiorelli S, Porro B, Cosentino N, Di Minno A, Manega CM, Fabbiocchi F, et al. Activation of Nrf2/HO-1 pathway and human atherosclerotic plaque vulnerability: an in vitro and in vivo study. Cells. 2019; 8:356.21. Cheng C, Noordeloos AM, Jeney V, Soares MP, Moll F, Pasterkamp G, et al. Heme oxygenase 1 determines atherosclerotic lesion progression into a vulnerable plaque. Circulation. 2009; 119:3017–27.22. Alonso-Pineiro JA, Gonzalez-Rovira A, Sanchez-Gomar I, Moreno JA, Duran-Ruiz MC. Nrf2 and heme oxygenase-1 involvement in atherosclerosis related oxidative stress. Antioxidants (Basel). 2021; 10:1463.23. Singh A, Venkannagari S, Oh KH, Zhang YQ, Rohde JM, Liu L, et al. Small molecule inhibitor of NRF2 selectively intervenes therapeutic resistance in KEAP1-deficient NSCLC tumors. ACS Chem Biol. 2016; 11:3214–25.24. Zeeshan HM, Lee GH, Kim HR, Chae HJ. Endoplasmic reticulum stress and associated ROS. Int J Mol Sci. 2016; 17:327.25. Ren J, Bi Y, Sowers JR, Hetz C, Zhang Y. Endoplasmic reticulum stress and unfolded protein response in cardiovascular diseases. Nat Rev Cardiol. 2021; 18:499–521.26. Myoishi M, Hao H, Minamino T, Watanabe K, Nishihira K, Hatakeyama K, et al. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation. 2007; 116:1226–33.27. Carlisle RE, Werner KE, Yum V, Lu C, Tat V, Memon M, et al. Endoplasmic reticulum stress inhibition reduces hypertension through the preservation of resistance blood vessel structure and function. J Hypertens. 2016; 34:1556–69.28. Ussher JR, Drucker DJ. Cardiovascular actions of incretinbased therapies. Circ Res. 2014; 114:1788–803.29. Kaku K, Enya K, Nakaya R, Ohira T, Matsuno R. Efficacy and safety of fasiglifam (TAK-875), a G protein-coupled receptor 40 agonist, in Japanese patients with type 2 diabetes inadequately controlled by diet and exercise: a randomized, double-blind, placebo-controlled, phase III trial. Diabetes Obes Metab. 2015; 17:675–81.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Endoplasmic Reticulum Stress and Diabetes
- Fasiglifam (TAK-875), a G Protein-Coupled Receptor 40 (GPR40) Agonist, May Induce Hepatotoxicity through Reactive Oxygen Species Generation in a GPR40-Dependent Manner
- Reactive oxygen species-mediated unfolded protein response pathways in preimplantation embryos
- Treatment with Phytoestrogens Reversed Triclosan and Bisphenol A-Induced Anti-Apoptosis in Breast Cancer Cells
- Cynaropicrin Induces Reactive Oxygen Species-Dependent Paraptosis-Like Cell Death in Human Liver Cancer Cells