Endocrinol Metab.
2023 Dec;38(6):739-749. 10.3803/EnM.2023.1780.
Phospholipase C-γ as a Potential Therapeutic Target for Graves’ Orbitopathy
- Affiliations
-
- 1Department of Medicine, Yonsei University College of Medicine, Seoul, Korea
- 2Department of Ophthalmology, Severance Hospital, Institute of Vision Research, Yonsei University College of Medicine, Seoul, Korea
- 3Division of Oculofacial Plastic and Reconstructive Surgery, Department of Ophthalmology, Shiley Eye Institute, University of California San Diego, La Jolla, CA, USA
- 4Department of Ophthalmology, Soonchunhyang University Bucheon Hospital, Soonchunhyang University College of Medicine, Bucheon, Korea
- KMID: 2549267
- DOI: http://doi.org/10.3803/EnM.2023.1780
Abstract
- Background
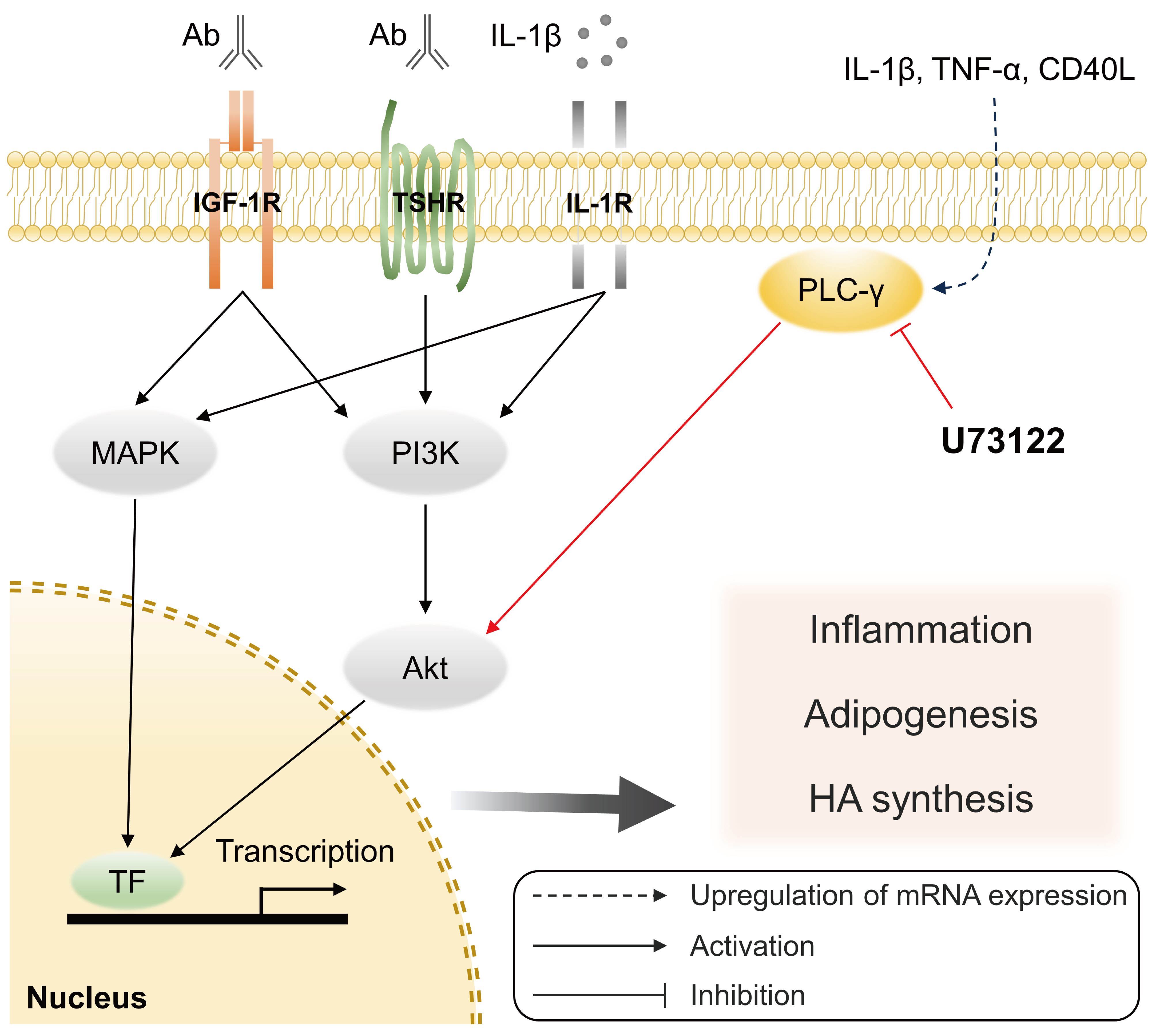
Phospholipase C-γ (PLC-γ) plays a crucial role in immune responses and is related to the pathogenesis of various inflammatory disorders. In this study, we investigated the role of PLC-γ and the therapeutic effect of the PLC-specific inhibitor U73122 using orbital fibroblasts from patients with Graves’ orbitopathy (GO).
Methods
The expression of phospholipase C gamma 1 (PLCG1) and phospholipase C gamma 2 (PLCG2) was evaluated using polymerase chain reaction in GO and normal orbital tissues/fibroblasts. The primary cultures of orbital fibroblasts were treated with non-toxic concentrations of U73122 with or without interleukin (IL)-1β to determine its therapeutic efficacy. The proinflammatory cytokine levels and activation of downstream signaling molecules were determined using Western blotting.
Results
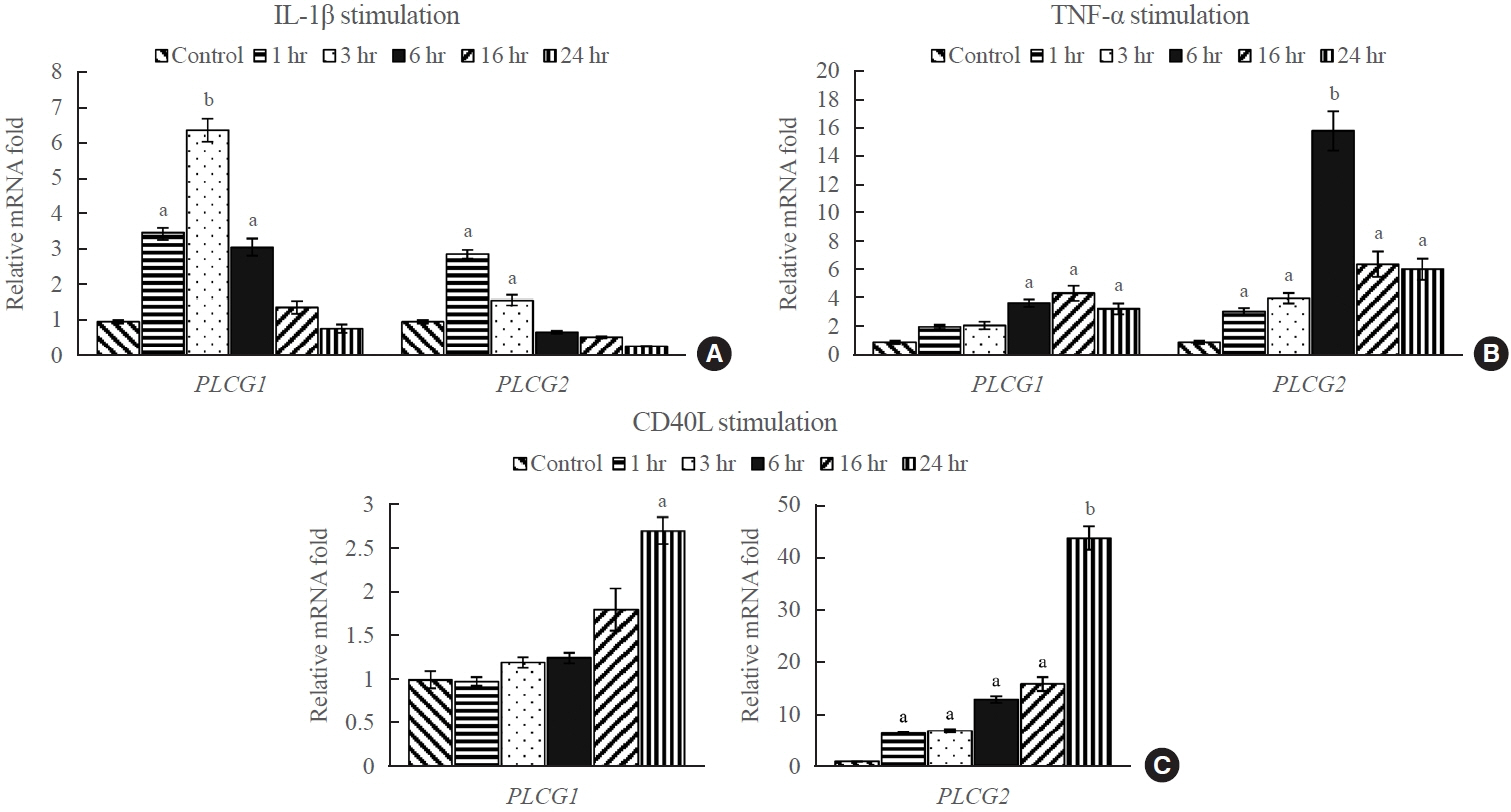
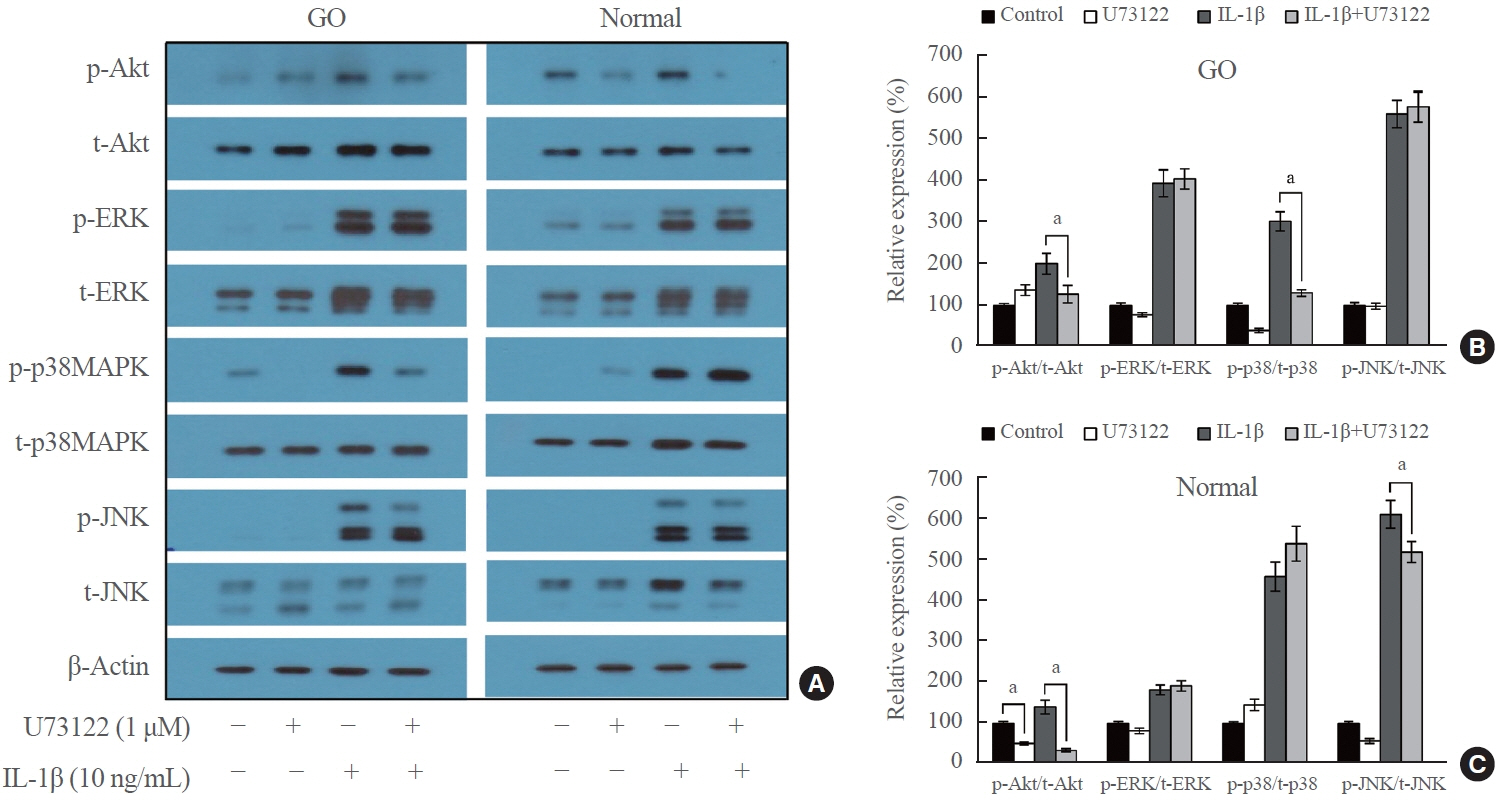
PLCG1 and PLCG2 mRNA expression was significantly higher in GO orbital tissues than in controls (P<0.05). PLCG1 and PLCG2 mRNA expression was significantly increased (P<0.05) in IL-1β, tumor necrosis factor-α, and a cluster of differentiation 40 ligand-stimulated GO fibroblasts. U73122 significantly inhibited the IL-1β-induced expression of proinflammatory molecules, including IL-6, IL-8, monocyte chemoattractant protein-1, cyclooxygenase-2, and intercellular adhesion molecule-1 (ICAM-1), and phosphorylated protein kinase B (p-Akt) and p38 (p-p38) kinase in GO fibroblasts, whereas it inhibited IL-6, IL-8, and ICAM-1, and p-Akt and c-Jun N-terminal kinase (p-JNK) in normal fibroblasts (P<0.05).
Conclusion
PLC-γ-inhibiting U73122 suppressed the production of proinflammatory cytokines and the phosphorylation of Akt and p38 kinase in GO fibroblasts. This study indicates the implications of PLC-γ in GO pathogenesis and its potential as a therapeutic target for GO.
Keyword
Figure
Reference
-
1. Wiersinga WM. Advances in treatment of active, moderateto-severe Graves’ ophthalmopathy. Lancet Diabetes Endocrinol. 2017; 5:134–42.
Article2. van Steensel L, Dik WA. The orbital fibroblast: a key player and target for therapy in graves’ ophthalmopathy. Orbit. 2010; 29:202–6.
Article3. Tsui S, Naik V, Hoa N, Hwang CJ, Afifiyan NF, Sinha Hikim A, et al. Evidence for an association between thyroidstimulating hormone and insulin-like growth factor 1 receptors: a tale of two antigens implicated in Graves’ disease. J Immunol. 2008; 181:4397–405.
Article4. Dik WA, Virakul S, van Steensel L. Current perspectives on the role of orbital fibroblasts in the pathogenesis of Graves’ ophthalmopathy. Exp Eye Res. 2016; 142:83–91.
Article5. Lehmann GM, Feldon SE, Smith TJ, Phipps RP. Immune mechanisms in thyroid eye disease. Thyroid. 2008; 18:959–65.
Article6. Bahn RS. Graves’ ophthalmopathy. N Engl J Med. 2010; 362:726–38.
Article7. Taylor PN, Zhang L, Lee RW, Muller I, Ezra DG, Dayan CM, et al. New insights into the pathogenesis and nonsurgical management of Graves orbitopathy. Nat Rev Endocrinol. 2020; 16:104–16.
Article8. Kadamur G, Ross EM. Mammalian phospholipase C. Annu Rev Physiol. 2013; 75:127–54.
Article9. Yang YR, Follo MY, Cocco L, Suh PG. The physiological roles of primary phospholipase C. Adv Biol Regul. 2013; 53:232–41.10. Koss H, Bunney TD, Behjati S, Katan M. Dysfunction of phospholipase Cγ in immune disorders and cancer. Trends Biochem Sci. 2014; 39:603–11.
Article11. Nakamura Y, Fukami K. Regulation and physiological functions of mammalian phospholipase C. J Biochem. 2017; 161:315–21.
Article12. Bae YS, Lee JH, Choi SH, Kim S, Almazan F, Witztum JL, et al. Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein: tolllike receptor 4- and spleen tyrosine kinase-dependent activation of NADPH oxidase 2. Circ Res. 2009; 104:210–8.13. Aki D, Minoda Y, Yoshida H, Watanabe S, Yoshida R, Takaesu G, et al. Peptidoglycan and lipopolysaccharide activate PLCgamma2, leading to enhanced cytokine production in macrophages and dendritic cells. Genes Cells. 2008; 13:199–208.14. Zhou Q, Lee GS, Brady J, Datta S, Katan M, Sheikh A, et al. A hypermorphic missense mutation in PLCG2, encoding phospholipase Cγ2, causes a dominantly inherited autoinflammatory disease with immunodeficiency. Am J Hum Genet. 2012; 91:713–20.15. Afroz S, Giddaluru J, Vishwakarma S, Naz S, Khan AA, Khan N. A comprehensive gene expression meta-analysis identifies novel immune signatures in rheumatoid arthritis patients. Front Immunol. 2017; 8:74.16. Zhu L, Yuan C, Ding X, Xu S, Yang J, Liang Y, et al. PLC-γ1 is involved in the inflammatory response induced by influenza A virus H1N1 infection. Virology. 2016; 496:131–7.17. Mandal S, Bandyopadhyay S, Tyagi K, Roy A. Recent advances in understanding the molecular role of phosphoinositide-specific phospholipase C gamma 1 as an emerging oncodriver and novel therapeutic target in human carcinogenesis. Biochim Biophys Acta Rev Cancer. 2021; 1876:188619.18. Yu P, Constien R, Dear N, Katan M, Hanke P, Bunney TD, et al. Autoimmunity and inflammation due to a gain-of-function mutation in phospholipase C gamma 2 that specifically increases external Ca2+ entry. Immunity. 2005; 22:451–65.19. Abe K, Fuchs H, Boersma A, Hans W, Yu P, Kalaydjiev S, et al. A novel N-ethyl-N-nitrosourea-induced mutation in phospholipase Cγ2 causes inflammatory arthritis, metabolic defects, and male infertility in vitro in a murine model. Arthritis Rheum. 2011; 63:1301–11.20. Cremasco V, Graham DB, Novack DV, Swat W, Faccio R. Vav/phospholipase Cgamma2-mediated control of a neutrophil-dependent murine model of rheumatoid arthritis. Arthritis Rheum. 2008; 58:2712–22.21. Cremasco V, Benasciutti E, Cella M, Kisseleva M, Croke M, Faccio R. Phospholipase C gamma 2 is critical for development of a murine model of inflammatory arthritis by affecting actin dynamics in dendritic cells. PLoS One. 2010; 5:e8909.22. Zhu L, Yuan C, Ma Y, Ding X, Zhu G, Zhu Q. Anti-inflammatory activities of phospholipase C inhibitor U73122: inhibition of monocyte-to-macrophage transformation and LPSinduced pro-inflammatory cytokine expression. Int Immunopharmacol. 2015; 29:622–7.23. Zhou Z, Xi R, Liu J, Peng X, Zhao L, Zhou X, et al. TAS2R16 activation suppresses LPS-induced cytokine expression in human gingival fibroblasts. Front Immunol. 2021; 12:726546.24. Yoon JS, Lee HJ, Choi SH, Chang EJ, Lee SY, Lee EJ. Quercetin inhibits IL-1β-induced inflammation, hyaluronan production and adipogenesis in orbital fibroblasts from Graves’ orbitopathy. PLoS One. 2011; 6:e26261.25. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001; 25:402–8.26. Jackson JT, Mulazzani E, Nutt SL, Masters SL. The role of PLCγ2 in immunological disorders, cancer, and neurodegeneration. J Biol Chem. 2021; 297:100905.27. Paul S, Schaefer BC. A new look at T cell receptor signaling to nuclear factor-κB. Trends Immunol. 2013; 34:269–81.28. Fu G, Chen Y, Yu M, Podd A, Schuman J, He Y, et al. Phospholipase C{gamma}1 is essential for T cell development, activation, and tolerance. J Exp Med. 2010; 207:309–18.29. de Gorter DJ, Vos JC, Pals ST, Spaargaren M. The B cell antigen receptor controls AP-1 and NFAT activity through Ras-mediated activation of Ral. J Immunol. 2007; 178:1405–14.30. Sempowski GD, Rozenblit J, Smith TJ, Phipps RP. Human orbital fibroblasts are activated through CD40 to induce proinflammatory cytokine production. Am J Physiol. 1998; 274:C707–14.31. Liu Z, Liu Y, Liu M, Gong Q, Shi A, Li X, et al. PD-L1 inhibits T cell-induced cytokines and hyaluronan expression via the CD40-CD40L pathway in orbital fibroblasts from patients with thyroid associated ophthalmopathy. Front Immunol. 2022; 13:849480.32. Hwang CJ, Afifiyan N, Sand D, Naik V, Said J, Pollock SJ, et al. Orbital fibroblasts from patients with thyroid-associated ophthalmopathy overexpress CD40: CD154 hyperinduces IL-6, IL-8, and MCP-1. Invest Ophthalmol Vis Sci. 2009; 50:2262–8.33. So L, Fruman DA. PI3K signalling in B- and T-lymphocytes: new developments and therapeutic advances. Biochem J. 2012; 442:465–81.34. Kumar S, Nadeem S, Stan MN, Coenen M, Bahn RS. A stimulatory TSH receptor antibody enhances adipogenesis via phosphoinositide 3-kinase activation in orbital preadipocytes from patients with Graves’ ophthalmopathy. J Mol Endocrinol. 2011; 46:155–63.35. Draman MS, Zhang L, Dayan C, Ludgate M. Orbital signaling in Graves’ orbitopathy. Front Endocrinol (Lausanne). 2021; 12:739994.36. Zhao P, Deng Y, Gu P, Wang Y, Zhou H, Hu Y, et al. Insulinlike growth factor 1 promotes the proliferation and adipogenesis of orbital adipose-derived stromal cells in thyroidassociated ophthalmopathy. Exp Eye Res. 2013; 107:65–73.37. Ko J, Kim JY, Lee EJ, Yoon JS. Inhibitory effect of idelalisib, a selective phosphatidylinositol 3-kinase δ inhibitor, on adipogenesis in an in vitro model of Graves’ orbitopathy. Invest Ophthalmol Vis Sci. 2018; 59:4477–85.38. Li D, Shatos MA, Hodges RR, Dartt DA. Role of PKCα activation of Src, PI-3K/AKT, and ERK in EGF-stimulated proliferation of rat and human conjunctival goblet cells. Invest Ophthalmol Vis Sci. 2013; 54:5661–74.39. Wu D, Peng F, Zhang B, Ingram AJ, Kelly DJ, Gilbert RE, et al. EGFR-PLCgamma1 signaling mediates high glucoseinduced PKCbeta1-Akt activation and collagen I upregulation in mesangial cells. Am J Physiol Renal Physiol. 2009; 297:F822–34.40. Amin AR, Ichigotani Y, Oo ML, Biswas MH, Yuan H, Huang P, et al. The PLC-PKC cascade is required for IL-1beta-dependent Erk and Akt activation: their role in proliferation. Int J Oncol. 2003; 23:1727–31.41. Colonna M. TREMs in the immune system and beyond. Nat Rev Immunol. 2003; 3:445–53.42. Chen MH, Chen MH, Liao SL, Chang TC, Chuang LM. Role of macrophage infiltration in the orbital fat of patients with Graves’ ophthalmopathy. Clin Endocrinol (Oxf). 2008; 69:332–7.43. Han SY, Choi SH, Shin JS, Lee EJ, Han SH, Yoon JS. High-mobility group box 1 is associated with the inflammatory pathogenesis of Graves’ orbitopathy. Thyroid. 2019; 29:868–78.44. Glassford J, Soeiro I, Skarell SM, Banerji L, Holman M, Klaus GG, et al. BCR targets cyclin D2 via Btk and the p85alpha subunit of PI3-K to induce cell cycle progression in primary mouse B cells. Oncogene. 2003; 22:2248–59.45. Lo Vasco VR, Leopizzi M, Di Maio V, Della Rocca C. U73122 reduces the cell growth in cultured MG-63 ostesarcoma cell line involving phosphoinositide-specific phospholipases C. Springerplus. 2016; 5:156.46. Peng T, Shen E, Fan J, Zhang Y, Arnold JM, Feng Q. Disruption of phospholipase Cgamma1 signalling attenuates cardiac tumor necrosis factor-alpha expression and improves myocardial function during endotoxemia. Cardiovasc Res. 2008; 78:90–7.47. Macmillan D, McCarron JG. The phospholipase C inhibitor U-73122 inhibits Ca(2+) release from the intracellular sarcoplasmic reticulum Ca(2+) store by inhibiting Ca(2+) pumps in smooth muscle. Br J Pharmacol. 2010; 160:1295–301.48. Klose A, Huth T, Alzheimer C. 1-[6-[[(17beta)-3-methoxyestra-1,3,5(10)-trien -17-yl]amino]hexyl]-1H-pyrrole-2,5-dione (U73122) selectively inhibits Kir3 and BK channels in a phospholipase C-independent fashion. Mol Pharmacol. 2008; 74:1203–14.49. Feisst C, Albert D, Steinhilber D, Werz O. The aminosteroid phospholipase C antagonist U-73122 (1-[6-[[17-beta-3-methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-1H-pyrrole-2, 5-dione) potently inhibits human 5-lipoxygenase in vivo and in vitro. Mol Pharmacol. 2005; 67:1751–7.50. Chen B, Tsui S, Smith TJ. IL-1 beta induces IL-6 expression in human orbital fibroblasts: identification of an anatomicsite specific phenotypic attribute relevant to thyroid-associated ophthalmopathy. J Immunol. 2005; 175:1310–9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of Inositol-Requiring Enzyme 1 and Autophagy in the Pro-Fibrotic Mechanism Underlying Graves’ Orbitopathy
- Radiation Therapy for Thyroid Orbitopathy
- The Clinical Characteristics of Thyroid Orbitopathy in Thyroid Dysfunction Pediatric Patients
- Serum Selenium Levels in Patients with Graves Disease: Associations with Clinical Activity and Severity in a Retrospective Case-control Study
- Erratum to "Correlation between TSH Receptor Antibody Assays and Clinical Manifestations of Graves' Orbitopathy" by Jang SY, et al. (Yonsei Med J 2013;54:1033-9.)