Endocrinol Metab.
2023 Dec;38(6):701-708. 10.3803/EnM.2023.1783.
Higher Plasma Stromal Cell-Derived Factor 1 Is Associated with Lower Risk for Sarcopenia in Older Asian Adults
- Affiliations
-
- 1Division of Geriatrics, Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Asan Institute for Life Sciences, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 3Department of Digital Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 4Department of Convergence Medicine, Asan Institute for Life Sciences, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 5Division of Endocrinology and Metabolism, Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2549263
- DOI: http://doi.org/10.3803/EnM.2023.1783
Abstract
- Background
Despite the protective effects of stromal cell-derived factor 1 (SDF-1) in stimulating muscle regeneration shown in experimental research, there is a lack of clinical studies linking circulating SDF-1 concentrations with muscle phenotypes. In order to elucidate the role of SDF-1 as a potential biomarker reflecting human muscle health, we investigated the association of plasma SDF-1 levels with sarcopenia in older adults.
Methods
This cross-sectional study included 97 community-dwelling participants who underwent a comprehensive geriatric assessment at a tertiary hospital in South Korea. Sarcopenia was defined by specific cutoff values applicable to the Asian population, whereas plasma SDF-1 levels were determined using an enzyme immunoassay.
Results
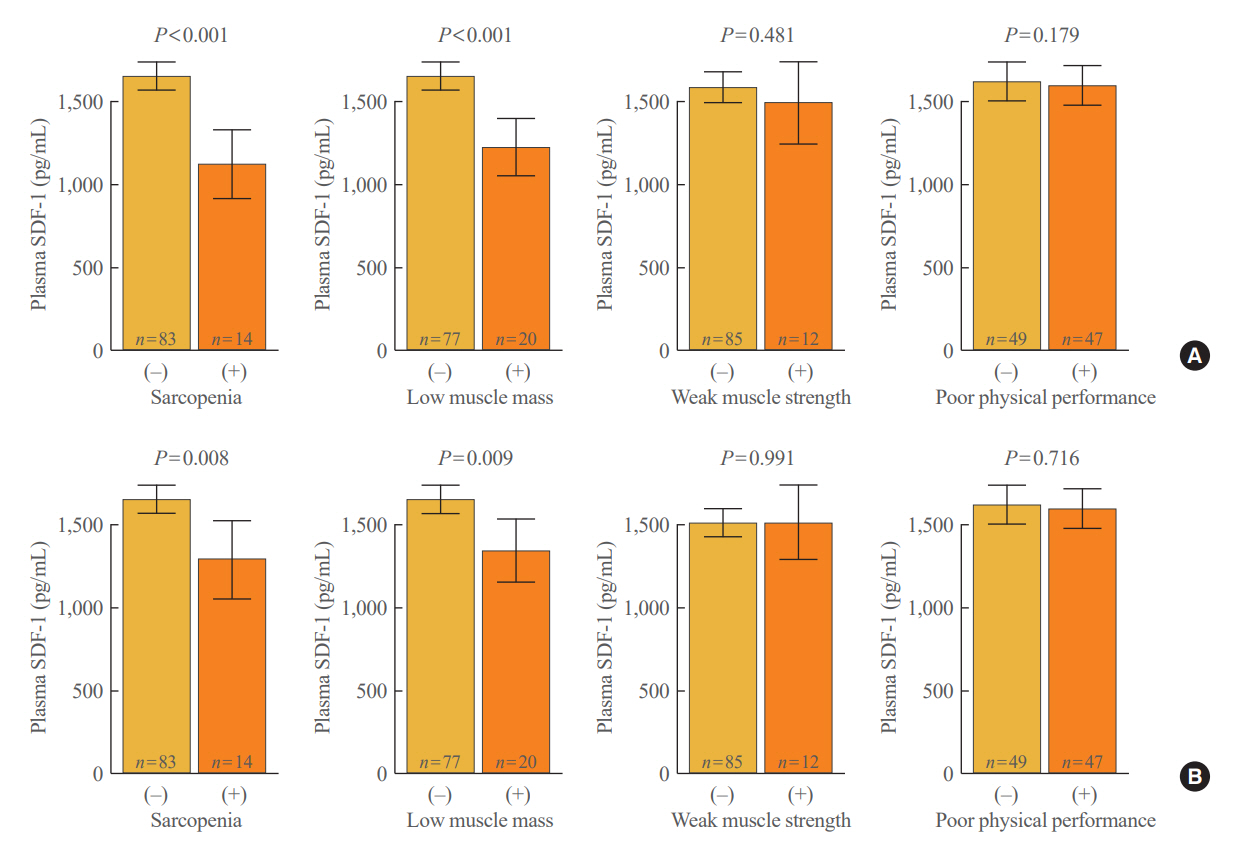
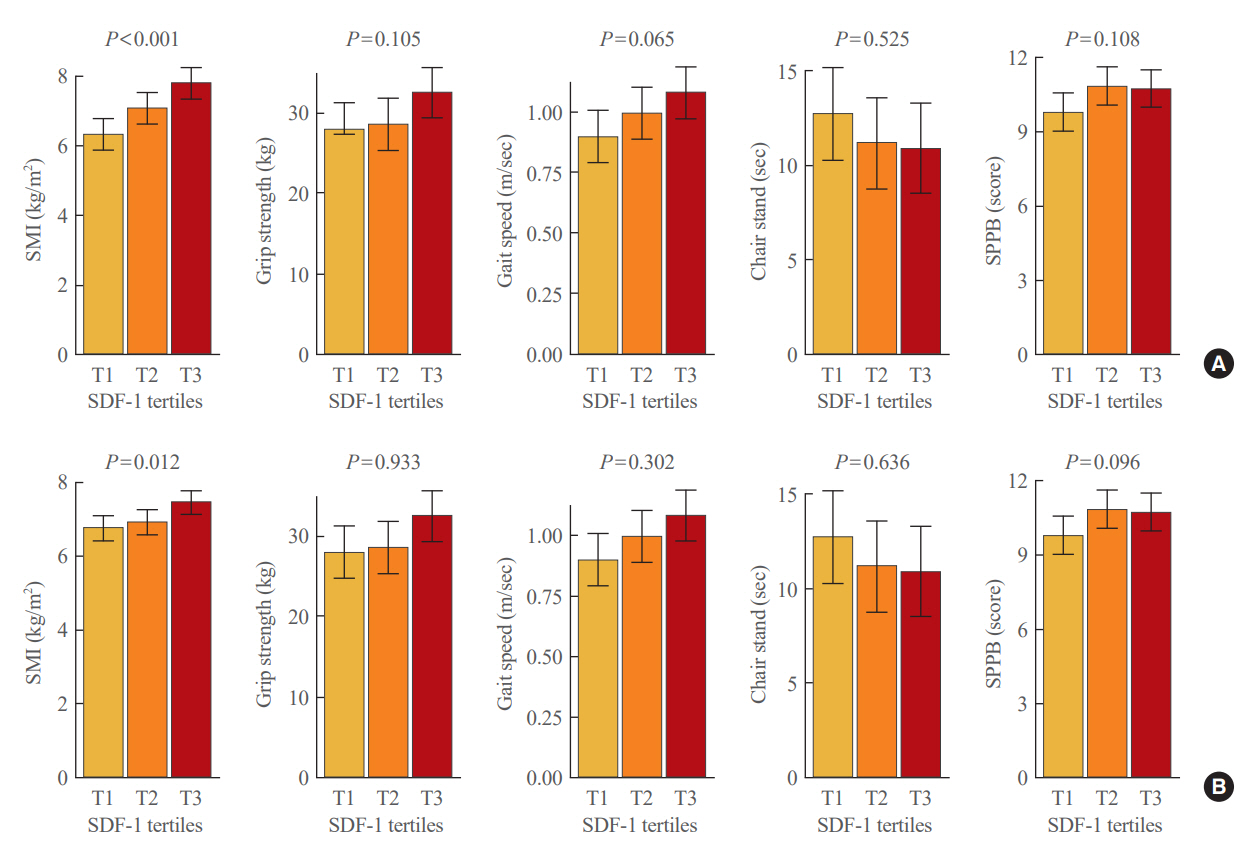
After accounting for sex, age, and body mass index, participants with sarcopenia and low muscle mass exhibited plasma SDF-1 levels that were 21.8% and 18.3% lower than those without these conditions, respectively (P=0.008 and P=0.009, respectively). Consistently, higher plasma SDF-1 levels exhibited a significant correlation with higher skeletal muscle mass index (SMI) and gait speed (both P=0.043), and the risk of sarcopenia and low muscle mass decreased by 58% and 55% per standard deviation increase in plasma SDF-1 levels, respectively (P=0.045 and P=0.030, respectively). Furthermore, participants in the highest SDF-1 tertile exhibited significantly higher SMI compared to those in the lowest tertile (P=0.012).
Conclusion
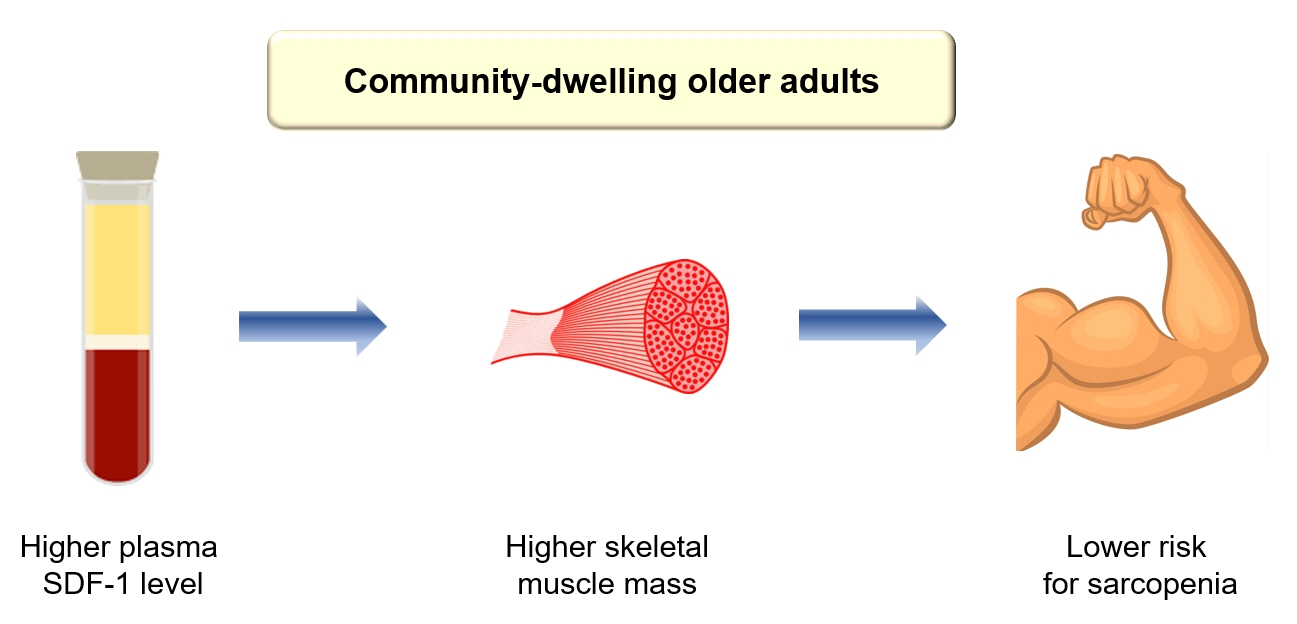
These findings clinically corroborate earlier experimental discoveries highlighting the muscle anabolic effects of SDF- 1 and support the potential role of circulating SDF-1 as a biomarker reflecting human muscle health in older adults.
Figure
Reference
-
1. Baek KW, Jung YK, Park JS, Kim JS, Hah YS, Kim SJ, et al. Two types of mouse models for sarcopenia research: senescence acceleration and genetic modification models. J Bone Metab. 2021; 28:179–91.
Article2. Baek JY, Jung HW, Kim KM, Kim M, Park CY, Lee KP, et al. Korean Working Group on Sarcopenia guideline: expert consensus on sarcopenia screening and diagnosis by the Korean Society of Sarcopenia, the Korean Society for Bone and Mineral Research, and the Korean Geriatrics Society. Ann Geriatr Med Res. 2023; 27:9–21.
Article3. Lee JY, Kim DA, Choi E, Lee YS, Park SJ, Kim BJ. Aldosterone inhibits in vitro myogenesis by increasing intracellular oxidative stress via mineralocorticoid receptor. Endocrinol Metab (Seoul). 2021; 36:865–74.4. Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. 2019; 393:2636–46.
Article5. Dao T, Green AE, Kim YA, Bae SJ, Ha KT, Gariani K, et al. Sarcopenia and muscle aging: a brief overview. Endocrinol Metab (Seoul). 2020; 35:716–32.
Article6. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019; 48:16–31.
Article7. Visser M, Schaap LA. Consequences of sarcopenia. Clin Geriatr Med. 2011; 27:387–99.
Article8. Sadri F, Rezaei Z, Fereidouni M. The significance of the SDF-1/CXCR4 signaling pathway in the normal development. Mol Biol Rep. 2022; 49:3307–20.
Article9. Schrader AJ, Lechner O, Templin M, Dittmar KE, Machtens S, Mengel M, et al. CXCR4/CXCL12 expression and signalling in kidney cancer. Br J Cancer. 2002; 86:1250–6.
Article10. Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1). J Exp Med. 1996; 184:1101–9.
Article11. Lau TT, Wang DA. Stromal cell-derived factor-1 (SDF-1): homing factor for engineered regenerative medicine. Expert Opin Biol Ther. 2011; 11:189–97.
Article12. Shiba Y, Takahashi M, Yoshioka T, Yajima N, Morimoto H, Izawa A, et al. M-CSF accelerates neointimal formation in the early phase after vascular injury in mice: the critical role of the SDF-1-CXCR4 system. Arterioscler Thromb Vasc Biol. 2007; 27:283–9.13. Psenak O. Stromal cell-derived factor 1 (SDF-1): its structure and function. Cas Lek Cesk. 2001; 140:355–63.14. Vasyutina E, Stebler J, Brand-Saberi B, Schulz S, Raz E, Birchmeier C. CXCR4 and Gab1 cooperate to control the development of migrating muscle progenitor cells. Genes Dev. 2005; 19:2187–98.15. Hunger C, Odemis V, Engele J. Expression and function of the SDF-1 chemokine receptors CXCR4 and CXCR7 during mouse limb muscle development and regeneration. Exp Cell Res. 2012; 318:2178–90.16. Melchionna R, Di Carlo A, De Mori R, Cappuzzello C, Barberi L, Musaro A, et al. Induction of myogenic differentiation by SDF-1 via CXCR4 and CXCR7 receptors. Muscle Nerve. 2010; 41:828–35.17. Brzoska E, Kowalewska M, Markowska-Zagrajek A, Kowalski K, Archacka K, Zimowska M, et al. Sdf-1 (CXCL12) improves skeletal muscle regeneration via the mobilisation of Cxcr4 and CD34 expressing cells. Biol Cell. 2012; 104:722–37.
Article18. Brzoska E, Kowalski K, Markowska-Zagrajek A, Kowalewska M, Archacki R, Plaskota I, et al. Sdf-1 (CXCL12) induces CD9 expression in stem cells engaged in muscle regeneration. Stem Cell Res Ther. 2015; 6:46.
Article19. Bobadilla M, Sainz N, Abizanda G, Orbe J, Rodriguez JA, Paramo JA, et al. The CXCR4/SDF1 axis improves muscle regeneration through MMP-10 activity. Stem Cells Dev. 2014; 23:1417–27.
Article20. Roberts HC, Denison HJ, Martin HJ, Patel HP, Syddall H, Cooper C, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011; 40:423–9.
Article21. Peel NM, Kuys SS, Klein K. Gait speed as a measure in geriatric assessment in clinical settings: a systematic review. J Gerontol A Biol Sci Med Sci. 2013; 68:39–46.22. Jung HW, Roh H, Cho Y, Jeong J, Shin YS, Lim JY, et al. Validation of a multi-sensor-based kiosk for short physical performance battery. J Am Geriatr Soc. 2019; 67:2605–9.23. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020; 21:300–7.24. Martinelli GB, Olivari D, Re Cecconi AD, Talamini L, Ottoboni L, Lecker SH, et al. Activation of the SDF1/CXCR4 pathway retards muscle atrophy during cancer cachexia. Oncogene. 2016; 35:6212–22.25. Tosato M, Marzetti E, Cesari M, Savera G, Miller RR, Bernabei R, et al. Measurement of muscle mass in sarcopenia: from imaging to biochemical markers. Aging Clin Exp Res. 2017; 29:19–27.26. Bhasin S, Travison TG, Manini TM, Patel S, Pencina KM, Fielding RA, et al. Sarcopenia definition: the position statements of the sarcopenia definition and outcomes consortium. J Am Geriatr Soc. 2020; 68:1410–8.27. Meza-Valderrama D, Marco E, Davalos-Yerovi V, Muns MD, Tejero-Sanchez M, Duarte E, et al. Sarcopenia, malnutrition, and cachexia: adapting definitions and terminology of nutritional disorders in older people with cancer. Nutrients. 2021; 13:761.28. Aleixo GF, Shachar SS, Nyrop KA, Muss HB, Battaglini CL, Williams GR. Bioelectrical impedance analysis for the assessment of sarcopenia in patients with cancer: a systematic review. Oncologist. 2020; 25:170–82.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sarcopenia, Frailty, and Diabetes in Older Adults
- The Relationship between Chronic Musculoskeletal Pain and Sarcopenia Risk in Community-Dwelling Older Adults: A Cross-Sectional Study
- Association between Possible Sarcopenia and Depressive Symptoms in Korean Older Adults: Results from the Korea National Health and Nutrition Examination Survey in 2018
- Case-Finding for Sarcopenia in Community-Dwelling Older Adults: Comparison of Mini Sarcopenia Risk Assessment with SARC-F and SARC-CalF
- How to Diagnose Sarcopenia in Korean Older Adults?