J Rhinol.
2023 Nov;30(3):161-166. 10.18787/jr.2023.00062.
The Relationship Between Zonulin and Asthma: A Mouse Model Study
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea
- 2Institute of Medical Research, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea
- 3Department of Pathology, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea
- 4Department of Pathology, Yuseong Sun Hospital, Daejeon, Republic of Korea
- 5Department of Pulmonology and Allergy, Hallym University Dongtan Sacred Heart Hospital, Hwaseong, Republic of Korea
- KMID: 2548194
- DOI: http://doi.org/10.18787/jr.2023.00062
Abstract
- Background and Objectives
Zonulin is a human protein that regulates intercellular tight junctions and increases the permeability of the intestinal epithelium. In light of the increasing focus on zonulin’s role in numerous chronic inflammatory diseases, this study aimed to investigate whether differences exist in serum zonulin levels and bronchial epithelium zonulin expression in vivo between asthma and normal groups, using a mouse model.
Methods
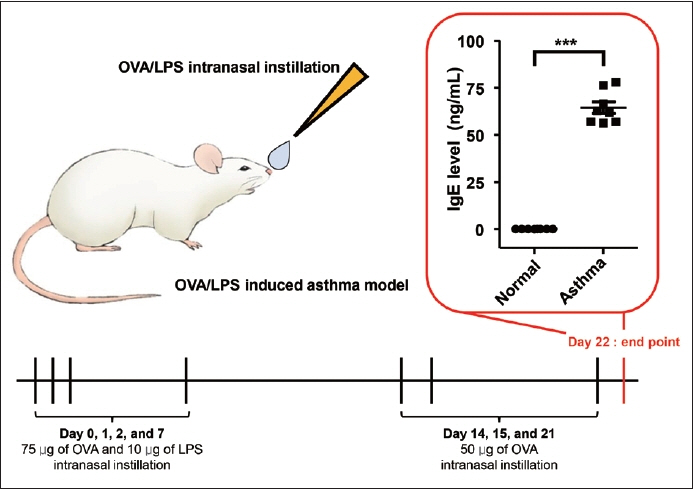
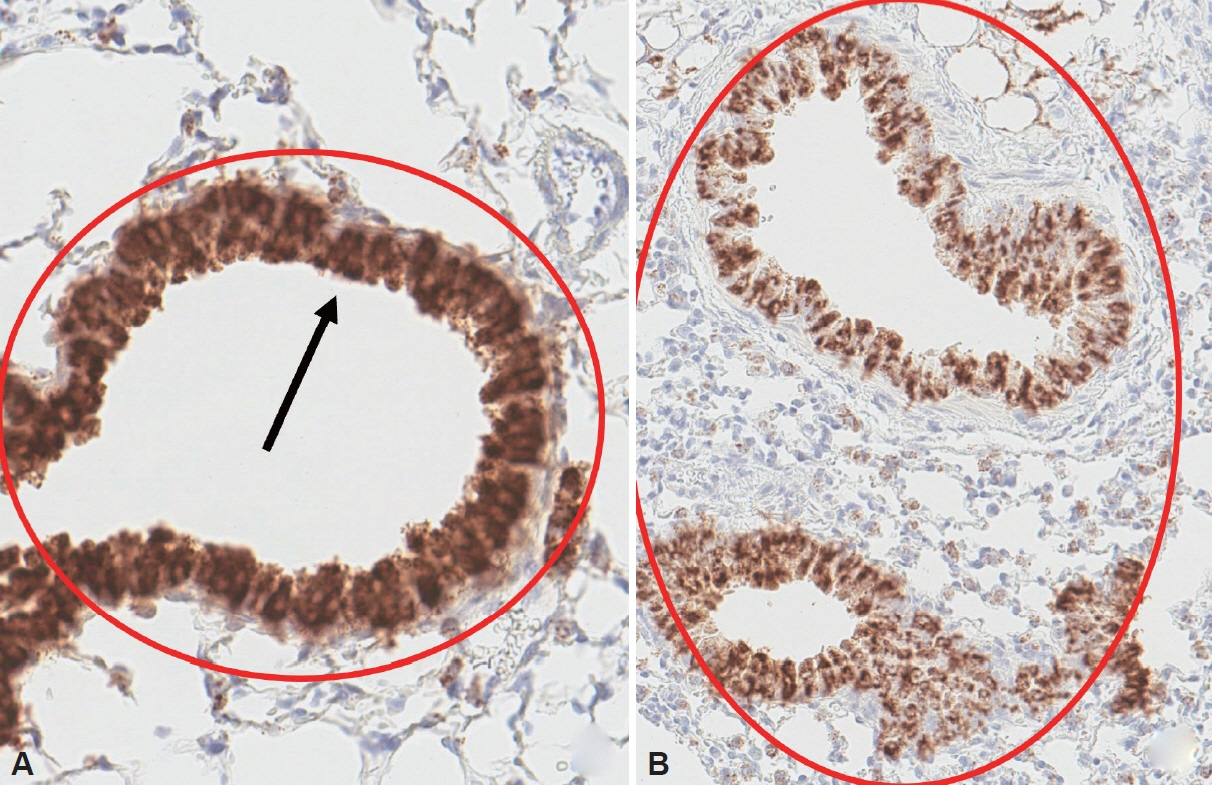
Sixteen mice were utilized in this study, divided evenly between the normal and asthma groups. Serum zonulin levels, the expression of zonulin antibody in the bronchial epithelium, and serum cytokine levels were evaluated in both groups. Enzyme-linked immunosorbent assay and RNA in situ hybridization were utilized for the analysis.
Results
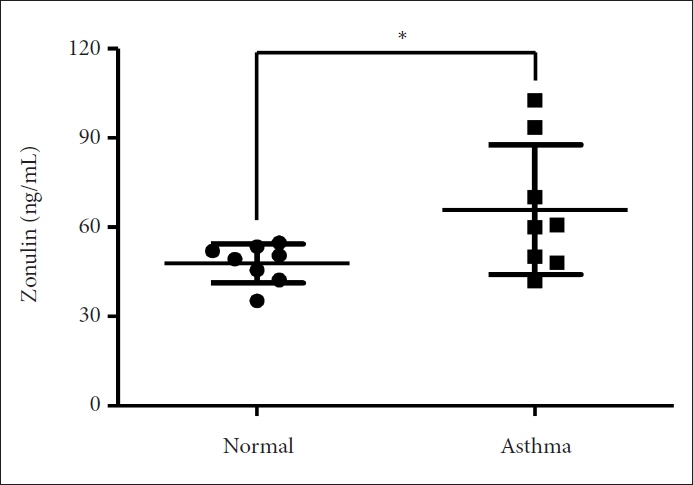
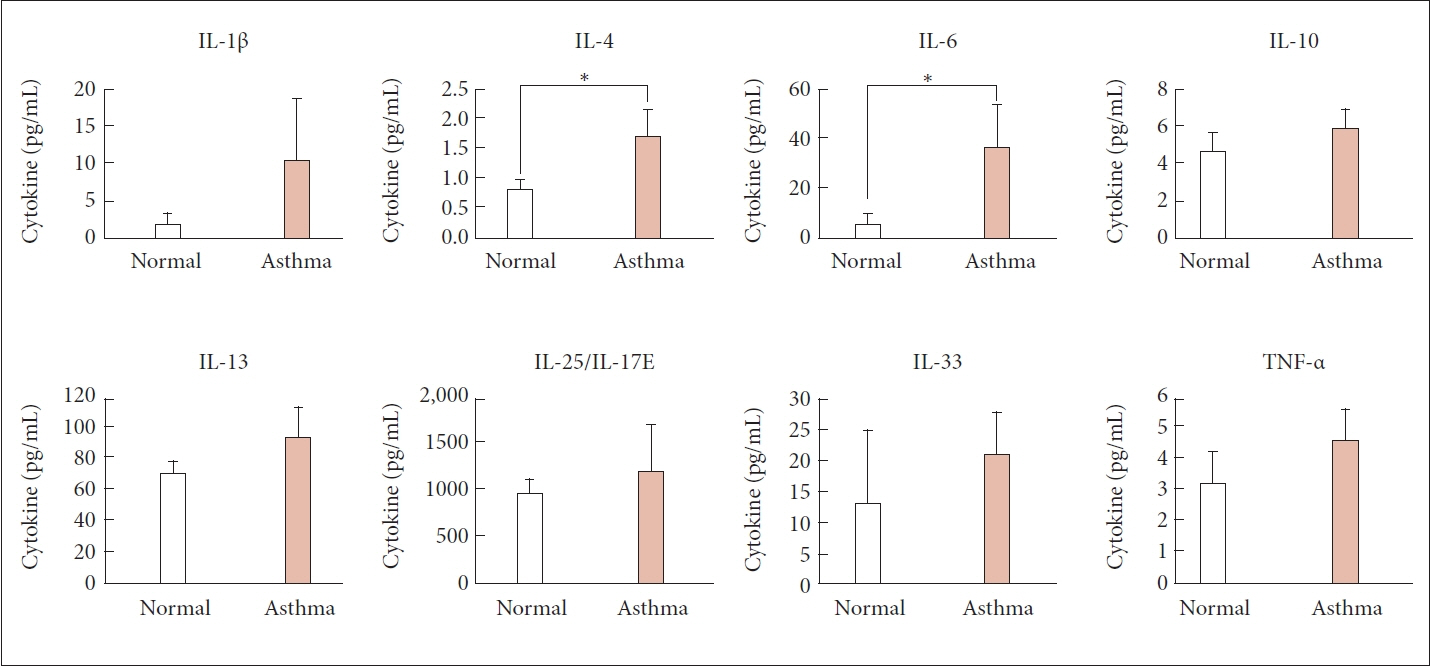
The asthma group exhibited significantly higher levels of serum zonulin. High zonulin antibody expression was also observed in the bronchial epithelium of the asthma group. Given that our mouse model demonstrated a significant difference in interleukin (IL)-4 and IL-6 between the normal and asthma groups, zonulin may be associated not only with type 2 responses but also with various subtypes of asthma. Further studies are required to investigate this relationship in greater detail.
Conclusion
Zonulin may play a role in the complex pathophysiology of asthma and could serve as a biomarker in various asthma-related situations.
Figure
Reference
-
References
1. Fasano A, Not T, Wang W, Uzzau S, Berti I, Tommasini A, et al. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet. 2000; 355(9214):1518–9.
Article2. Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev. 2011; 91(1):151–75.
Article3. Fasano A. All disease begins in the (leaky) gut: role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Res. 2020; 9(F1000 Faculty Rev):69.
Article4. Hijazi Z, Molla AM, Al-Habashi H, Muawad WM, Molla AM, Sharma PN. Intestinal permeability is increased in bronchial asthma. Arch Dis Child. 2004; 89(3):227–9.
Article5. Benard A, Desreumeaux P, Huglo D, Hoorelbeke A, Tonnel AB, Wallaert B. Increased intestinal permeability in bronchial asthma. J Allergy Clin Immunol. 1996; 97(6):1173–8.
Article6. Baioumy SA, Elgendy A, Ibrahim SM, Taha SI, Fouad SH. Association between serum zonulin level and severity of house dust mite allergic asthma. Allergy Asthma Clin Immunol. 2021; 17(1):86.
Article7. Kim NY, Shin E, Byeon SJ, Hong SJ, Kang SH, Lee T, et al. Serum zonulin is a biomarker for severe asthma. Allergy Asthma Immunol Res. 2023; 15(4):526–35.
Article8. Rehnberg M, Ramnegård M, Krutrök N, Yrlid L, Jirholt J. The role of lL-17 in the OVA-LPS driven model of lung inflammation. Eur Respir J. 2015; 46(suppl 59):PA4010.
Article9. Lambrecht BN, Hammad H, Fahy JV. The cytokines of asthma. Immunity. 2019; 50(4):975–91.
Article10. Heijink IH, Kuchibhotla VNS, Roffel MP, Maes T, Knight DA, Sayers I, et al. Epithelial cell dysfunction, a major driver of asthma development. Allergy. 2020; 75(8):1902–17.
Article11. Hellings PW, Steelant B. Epithelial barriers in allergy and asthma. J Allergy Clin Immunol. 2020; 145(6):1499–509.
Article12. Niewiem M, Grzybowska-Chlebowczyk U. Intestinal barrier permeability in allergic diseases. Nutrients. 2022; 14(9):1893.
Article13. Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, Serino M, et al. Intestinal permeability--a new target for disease prevention and therapy. BMC Gastroenterol. 2014; 14:189.14. Mouchati C, Durieux JC, Zisis SN, Labbato D, Rodgers MA, Ailstock K, et al. Increase in gut permeability and oxidized ldl is associated with post-acute sequelae of SARS-CoV-2. Front Immunol. 2023; 14:1182544.
Article15. Heidt C, Kämmerer U, Fobker M, Rüffer A, Marquardt T, Reuss-Borst M. Assessment of intestinal permeability and inflammation biomarkers in patients with rheumatoid arthritis. Nutrients. 2023; 15(10):2386.
Article16. Wasiak J, Gawlik-Kotelnicka O. Intestinal permeability and its significance in psychiatric disorders – A narrative review and future perspectives. Behav Brain Res. 2023; 448:114459.
Article17. Corren J, Busse W, Meltzer EO, Mansfield L, Bensch G, Fahrenholz J, et al. A randomized, controlled, phase 2 study of AMG 317, an IL-4Rα antagonist, in patients with asthma. Am J Respir Crit Care Med. 2010; 181(8):788–96.
Article18. Jensen ME, Gibson PG, Collins CE, Wood LG. Airway and systemic inflammation in obese children with asthma. Eur Respir J. 2013; 42(4):1012–9.
Article19. Zein JG, Dweik RA, Comhair SA, Bleecker ER, Moore WC, Peters SP, et al. Asthma is more severe in older adults. PLoS One. 2015; 10(7):e0133490.
Article20. Peters MC, McGrath KW, Hawkins GA, Hastie AT, Levy BD, Israel E, et al. Plasma interleukin-6 concentrations, metabolic dysfunction, and asthma severity: a cross-sectional analysis of two cohorts. Lancet Respir Med. 2016; 4(7):574–84.
Article21. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017; 377(12):1119–31.
Article22. Inoue K, Takano H, Yanagisawa R, Sakurai M, Shimada A, Sato M, et al. Role of interleukin-6 in fibrinolytic changes induced by lipopolysaccharide in mice. Blood Coagul Fibrinolysis. 2006; 17(4):307–9.
Article23. Inoue K, Takano H, Yanagisawa R, Sakurai M, Shimada A, Morita T, et al. Protective role of interleukin-6 in coagulatory and hemostatic disturbance induced by lipopolysaccharide in mice. Thromb Haemost. 2004; 91(6):1194–201.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of Serum Zonulin and Occludin Levels in Bipolar Disorder
- Understanding asthma using animal models
- Immunological pathogenesis of asthma in a mouse model Can asthma pathogenesis be explained by allergic reaction?
- Understanding the Mouse Model of Respiratory Allergic Diseases
- Mouse Model of Bronchial Asthma