Restor Dent Endod.
2021 May;46(2):e20. 10.5395/rde.2021.46.e20.
A novel antimicrobial-containing nanocellulose scaffold for regenerative endodontics
- Affiliations
-
- 1Department of Endodontics, Faculty of Dentistry, University of Southern Santa Catarina, Palhoça, SC, Brazil
- 2Department of Chemical Engineering, Federal University of Santa Catarina, Florianópolis, SC, Brazil
- KMID: 2548064
- DOI: http://doi.org/10.5395/rde.2021.46.e20
Abstract
Objectives
The aim of this study was to evaluate bacterial nanocellulose (BNC) membranes incorporated with antimicrobial agents regarding cytotoxicity in fibroblasts of the periodontal ligament (PDLF), antimicrobial activity, and inhibition of multispecies biofilm formation.
Materials and Methods
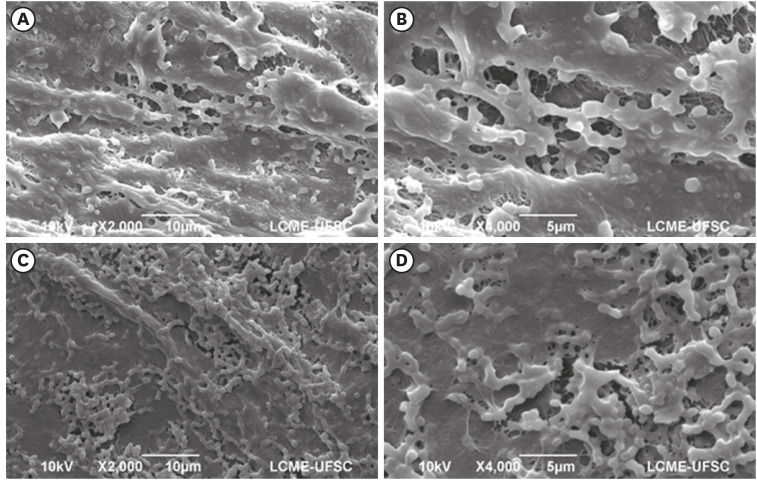
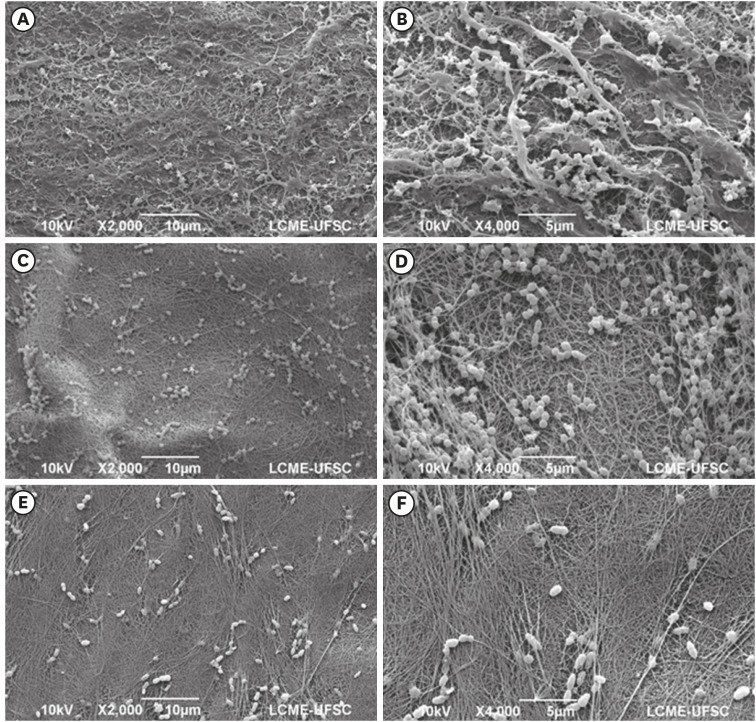
The tested BNC membranes were BNC + 1% clindamycin (BNC/CLI); BNC + 0.12% chlorhexidine (BNC/CHX); BNC + nitric oxide (BNC/NO); and conventional BNC (BNC; control). After PDLF culture, the BNC membranes were positioned in the wells and maintained for 24 hours. Cell viability was then evaluated using the MTS calorimetric test. Antimicrobial activity against Enterococcus faecalis, Actinomyces naeslundii, and Streptococcus sanguinis (S. sanguinis) was evaluated using the agar diffusion test. To assess the antibiofilm activity, BNC membranes were exposed for 24 hours to the mixed culture. After sonicating the BNC membranes to remove the remaining biofilm and plating the suspension on agar, the number of colony-forming units (CFU)/mL was determined. Data were analyzed by 1-way analysis of variance and the Tukey, Kruskal-Wallis, and Dunn tests (α = 5%).
Results
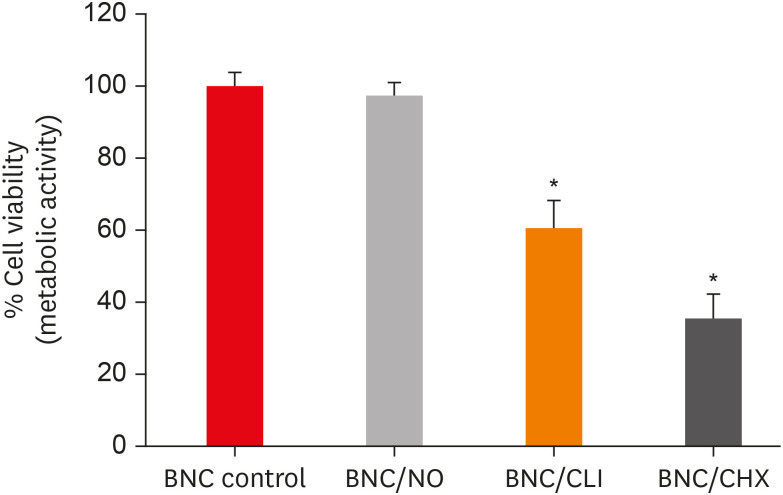
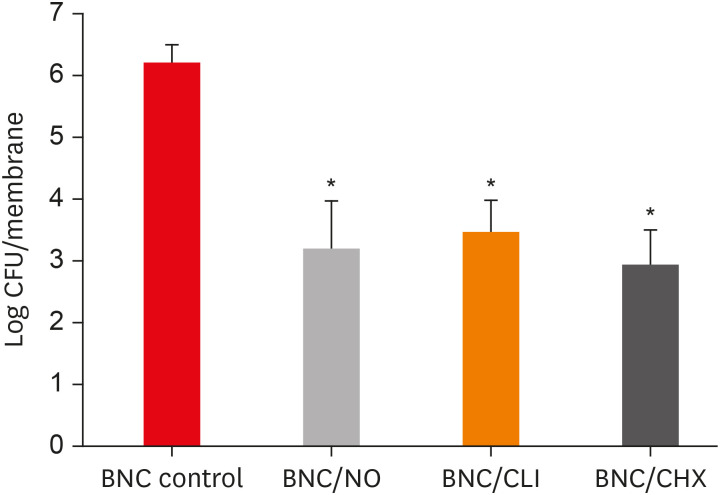
PDLF metabolic activity after contact with BNC/CHX, BNC/CLI, and BNC/NO was 35%, 61% and 97%, respectively, compared to BNC. BNC/NO showed biocompatibility similar to that of BNC (p = 0.78). BNC/CLI showed the largest inhibition halos, and was superior to the other BNC membranes against S. sanguinis (p < 0.05). The experimental BNC membranes inhibited biofilm formation, with about a 3-fold log CFU reduction compared to BNC (p < 0.05).
Conclusions
BNC/NO showed excellent biocompatibility and inhibited multispecies biofilm formation, similarly to BNC/CLI and BNC/CHX.
Figure
Reference
-
1. Jeeruphan T, Jantarat J, Yanpiset K, Suwannapan L, Khewsawai P, Hargreaves KM. Mahidol study 1: comparison of radiographic and survival outcomes of immature teeth treated with either regenerative endodontic or apexification methods: a retrospective study. J Endod. 2012; 38:1330–1336. PMID: 22980172.
Article2. Diogenes A, Ruparel NB, Shiloah Y, Hargreaves KM. Regenerative endodontics: a way forward. J Am Dent Assoc. 2016; 147:372–380. PMID: 27017182.3. Verma P, Nosrat A, Kim JR, Price JB, Wang P, Bair E, Xu HH, Fouad AF. Effect of residual bacteria on the outcome of pulp regeneration in vivo . J Dent Res. 2017; 96:100–106. PMID: 27694153.
Article4. Vishwanat L, Duong R, Takimoto K, Phillips L, Espitia CO, Diogenes A, Ruparel SB, Kolodrubetz D, Ruparel NB. Effect of bacterial biofilm on the osteogenic differentiation of stem cells of apical papilla. J Endod. 2017; 43:916–922. PMID: 28416302.
Article5. Curvello R, Raghuwanshi VS, Garnier G. Engineering nanocellulose hydrogels for biomedical applications. Adv Colloid Interface Sci. 2019; 267:47–61. PMID: 30884359.
Article6. Vismara E, Bernardi A, Bongio C, Farè S, Pappalardo S, Serafini A, Pollegioni L, Rosini E, Torri G. Bacterial nanocellulose and its surface modification by glycidyl methacrylate and ethylene glycol dimethacrylate. Incorporation of vancomycin and ciprofloxacin. Nanomaterials (Basel). 2019; 9:1668.
Article7. Osorio M, Ortiz I, Gañán P, Naranjo T, Zuluaga R, van Kooten TG, Castro C. Novel surface modification of three-dimensional bacterial nanocellulose with cell-derived adhesion proteins for soft tissue engineering. Mater Sci Eng C. 2019; 100:697–705.
Article8. Bottino MC, Pankajakshan D, Nör JE. Advanced scaffolds for dental pulp and periodontal regeneration. Dent Clin North Am. 2017; 61:689–711. PMID: 28886764.
Article9. Osorio M, Fernández-Morales P, Gañán P, Zuluaga R, Kerguelen H, Ortiz I, Castro C. Development of novel three-dimensional scaffolds based on bacterial nanocellulose for tissue engineering and regenerative medicine: effect of processing methods, pore size, and surface area. J Biomed Mater Res A. 2019; 107:348–359. PMID: 30421501.
Article10. Reis EMD, Berti FV, Colla G, Porto LM. Bacterial nanocellulose-IKVAV hydrogel matrix modulates melanoma tumor cell adhesion and proliferation and induces vasculogenic mimicry in vitro . J Biomed Mater Res B Appl Biomater. 2018; 106:2741–2749. PMID: 29206331.
Article11. Shoda M, Sugano Y. Recent advances in bacterial cellulose production. Biotechnol Bioprocess Eng. 2005; 10:1–8.
Article12. Dobmeier KP, Schoenfisch MH. Antibacterial properties of nitric oxide-releasing sol-gel microarrays. Biomacromolecules. 2004; 5:2493–2495. PMID: 15530068.
Article13. Seabra AB, Martins D, Simões MM, da Silva R, Brocchi M, de Oliveira MG. Antibacterial nitric oxide-releasing polyester for the coating of blood-contacting artificial materials. Artif Organs. 2010; 34:E204–E214. PMID: 20497163.
Article14. Moon CY, Nam OH, Kim M, Lee HS, Kaushik SN, Cruz Walma DA, Jun HW, Cheon K, Choi SC. Effects of the nitric oxide releasing biomimetic nanomatrix gel on pulp-dentin regeneration: pilot study. PLoS One. 2018; 13:e0205534. PMID: 30308037.
Article15. Kim JO, Noh JK, Thapa RK, Hasan N, Choi M, Kim JH, Lee JH, Ku SK, Yoo JW. Nitric oxide-releasing chitosan film for enhanced antibacterial and in vivo wound-healing efficacy. Int J Biol Macromol. 2015; 79:217–225. PMID: 25957720.
Article16. Pankajakshan D, Albuquerque MT, Evans JD, Kamocka MM, Gregory RL, Bottino MC. Triple antibiotic polymer nanofibers for intracanal drug delivery: effects on dual species biofilm and cell function. J Endod. 2016; 42:1490–1495. PMID: 27663615.
Article17. Karczewski A, Feitosa SA, Hamer EI, Pankajakshan D, Gregory RL, Spolnik KJ, Bottino MC. Clindamycin-modified triple antibiotic nanofibers: a stain-free antimicrobial intracanal drug delivery system. J Endod. 2018; 44:155–162. PMID: 29061356.
Article18. Gomes BP, Vianna ME, Zaia AA, Almeida JFA, Souza-Filho FJ, Ferraz CCR. Chlorhexidine in endodontics. Braz Dent J. 2013; 24:89–102. PMID: 23780357.
Article19. Galler KM, Buchalla W, Hiller KA, Federlin M, Eidt A, Schiefersteiner M, Schmalz G. Influence of root canal disinfectants on growth factor release from dentin. J Endod. 2015; 41:363–368. PMID: 25595468.
Article20. Widbiller M, Althumairy RI, Diogenes A. Direct and indirect effect of chlorhexidine on survival of stem cells from the apical papilla and its neutralization. J Endod. 2019; 45:156–160. PMID: 30711171.
Article21. De Souza SS, Berti FV, Oliveira KPV, Pittella C, Vasconcellos J, Pelissari C, Rambo CR, Porto LM. Nanocellulose biosynthesis by Komagataeibacter hansenii in a defined minimal culture medium. Cellulose. 2018; 26:1641–1655.
Article22. Lourenço SDM, de Oliveira MG. Topical photochemical nitric oxide release from porous poly(vinyl alcohol) membrane for visible light modulation of dermal vasodilation. J Photochem Photobiol Chem. 2017; 346:548–558.
Article23. Kumar V, Yang T. HNO3/H3PO4-NANO2 mediated oxidation of cellulose - preparation and characterization of bioabsorbable oxidized celluloses in high yields and with different levels of oxidation. Carbohydr Polym. 2002; 48:403–412.
Article24. Pittela CQP, Porto LM. Application of bacterial nanocellulose membranes for epithelial tissue repair. Revista de Enfermagem da UFJF. 2015; 1:223–232.25. Osorio M, Cañas A, Puerta J, Díaz L, Naranjo T, Ortiz I, Castro C. Ex vivo and in vivo biocompatibility assessment (blood and tissue) of three-dimensional bacterial nanocellulose biomaterials for soft tissue implants. Sci Rep. 2019; 9:10553. PMID: 31332259.26. Albuquerque MTP, Nagata J, Bottino MC. Antimicrobial efficacy of triple antibiotic-eluting polymer nanofibers against multispecies biofilm. J Endod. 2017; 43:S51–S56. PMID: 28778504.
Article27. Sun B, Slomberg DL, Chudasama SL, Lu Y, Schoenfisch MH. Nitric oxide-releasing dendrimers as antibacterial agents. Biomacromolecules. 2012; 13:3343–3354. PMID: 23013537.
Article28. Frost MC, Batchelor MM, Lee YM, Zhang H, Kang Y, Oh B, Wilson GS, Gifford R, Rudich SM, Meyerhoff M. Preparation and characterization of implantable sensors with nitric oxide release coatings. Microchem J. 2003; 74:277–288.
Article29. Sadrearhami Z, Nguyen TK, Namivandi-Zangeneh R, Jung K, Wong EHH, Boyer C. Recent advances in nitric oxide delivery for antimicrobial applications using polymer-based systems. J Mater Chem B. 2018; 6:2945–2959. PMID: 32254331.
Article30. Chuensombat S, Khemaleelakul S, Chattipakorn S, Srisuwan T. Cytotoxic effects and antibacterial efficacy of a 3-antibiotic combination: an in vitro study. J Endod. 2013; 39:813–819. PMID: 23683284.
Article31. Tanase S, Tsuchiya H, Yao J, Ohmoto S, Takagi N, Yoshida S. Reversed-phase ion-pair chromatographic analysis of tetracycline antibiotics. Application to discolored teeth. J Chromatogr B Biomed Sci Appl. 1998; 706:279–285. PMID: 9551814.
Article32. Rahhal JG, Rovai ED, Holzhausen M, Caldeira CL, Santos CF, Sipert CR. Root canal dressings for revascularization influence in vitro mineralization of apical papilla cells. J Appl Oral Sci. 2019; 27:e20180396. PMID: 30994774.
Article33. Zargar N, Rayat Hosein Abadi M, Sabeti M, Yadegari Z, Akbarzadeh Baghban A, Dianat O. Antimicrobial efficacy of clindamycin and triple antibiotic paste as root canal medicaments on tubular infection: an in vitro study. Aust Endod J. 2019; 45:86–91. PMID: 30113736.
Article34. Skucaite N, Peciuliene V, Vitkauskiene A, Machiulskiene V. Susceptibility of endodontic pathogens to antibiotics in patients with symptomatic apical periodontitis. J Endod. 2010; 36:1611–1616. PMID: 20850663.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Platelet rich fibrin - a novel acumen into regenerative endodontic therapy
- Biocompatibility of two experimental scaffolds for regenerative endodontics
- Antimicrobial effect of calcium hydroxide as an intracanal medicament in root canal treatment: a literature review - Part I. In vitro studies
- Antimicrobial Activity of Calcium Hydroxide in Endodontics: A Review
- Antimicrobial effect of calcium hydroxide as an intracanal medicament in root canal treatment: a literature review - Part II. in vivo studies