Restor Dent Endod.
2021 Feb;46(1):e4. 10.5395/rde.2021.46.e4.
Biocompatibility and bioactive potential of the NeoMTA Plus endodontic bioceramic-based sealer
- Affiliations
-
- 1Department of Restorative Dentistry, Dental School, São Paulo State University (UNESP), Araraquara, SP, Brazil
- 2Pro-Rectory of Research and Post-graduation, School of Dentistry, Universidade Sagrado Coração (USC), Bauru, SP, Brazil
- 3Department of Morphology, Genetics, Orthodontics and Pediatric Dentistry, Laboratory of Histology and Embryology, Dental School, São Paulo State University (UNESP), Araraquara, SP, Brazil
- KMID: 2548054
- DOI: http://doi.org/10.5395/rde.2021.46.e4
Abstract
Objectives
This study evaluated the biocompatibility and bioactive potential of NeoMTA Plus mixed as a root canal sealer in comparison with MTA Fillapex.
Materials and Methods
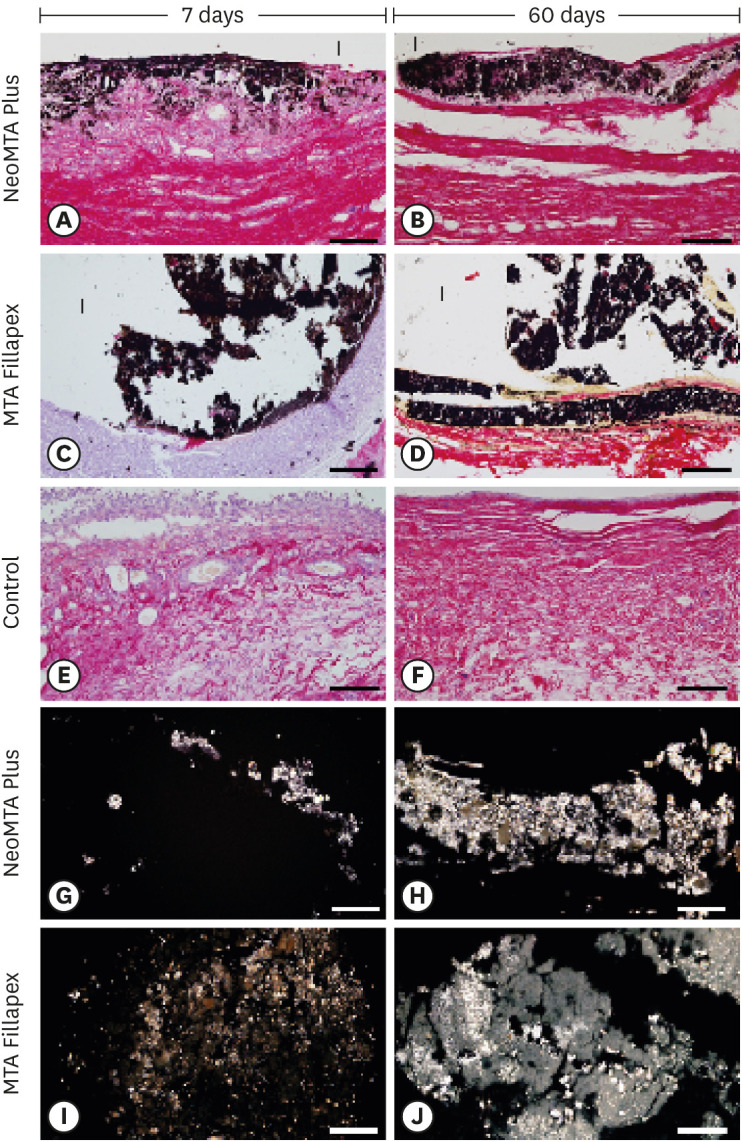
Polyethylene tubes filled with NeoMTA Plus (n = 20), MTA Fillapex (n = 20), or nothing (control group, CG; n = 20) were inserted into the connective tissue in the dorsal subcutaneous layer of rats. After 7, 15, 30 and 60 days, the specimens were processed for paraffin embedding. The capsule thickness, collagen content, and number of inflammatory cells (ICs) and interleukin-6 (IL-6) immunolabeled cells were measured. von Kossa-positive structures were evaluated and unstained sections were analyzed under polarized light. Two-way analysis of variance was performed, followed by the post hoc Tukey test (p ≤ 0.05).
Results
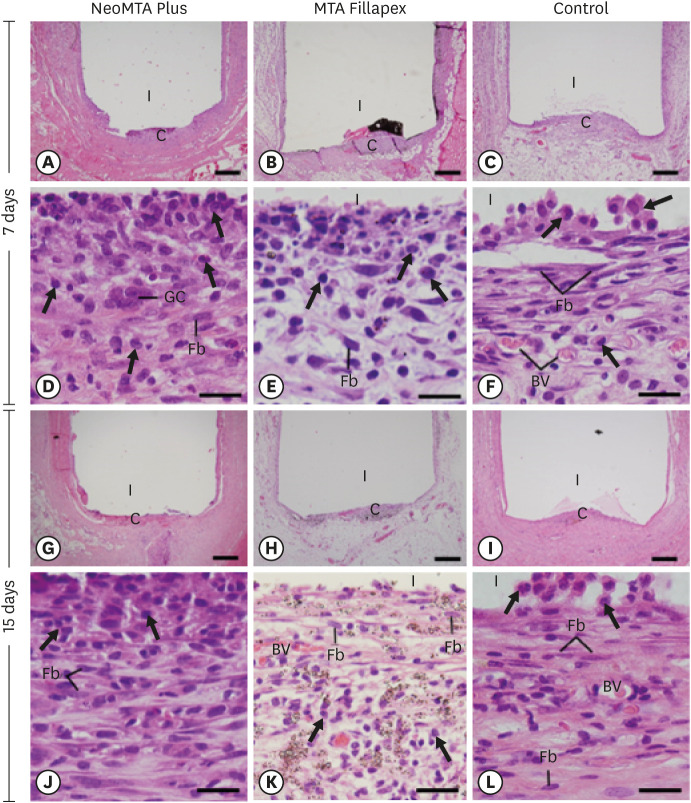
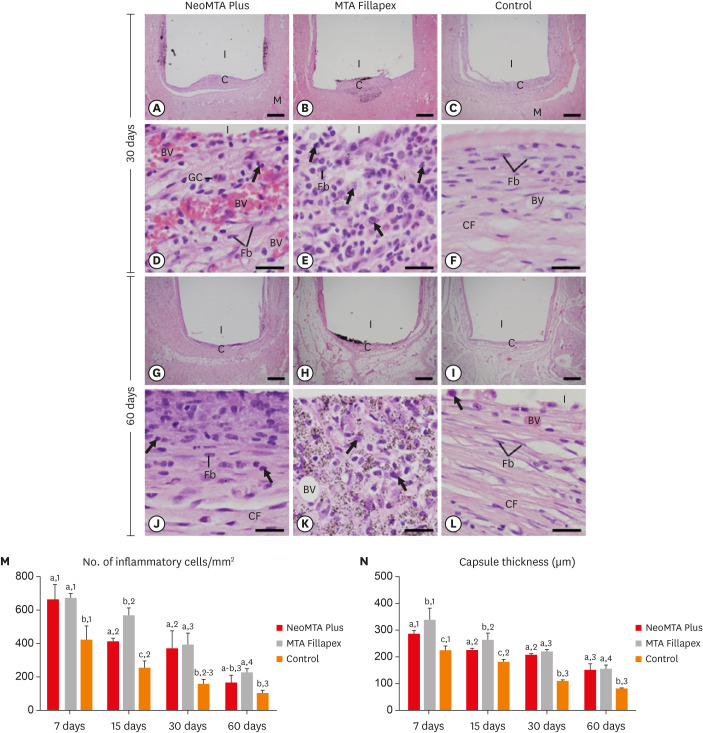
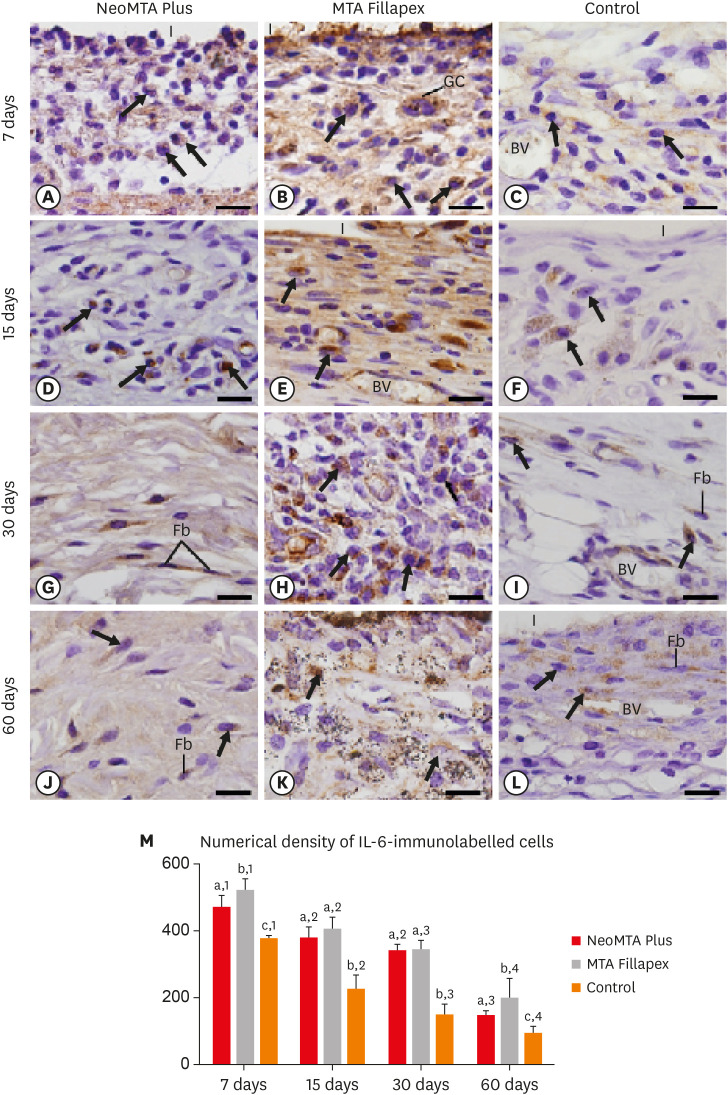
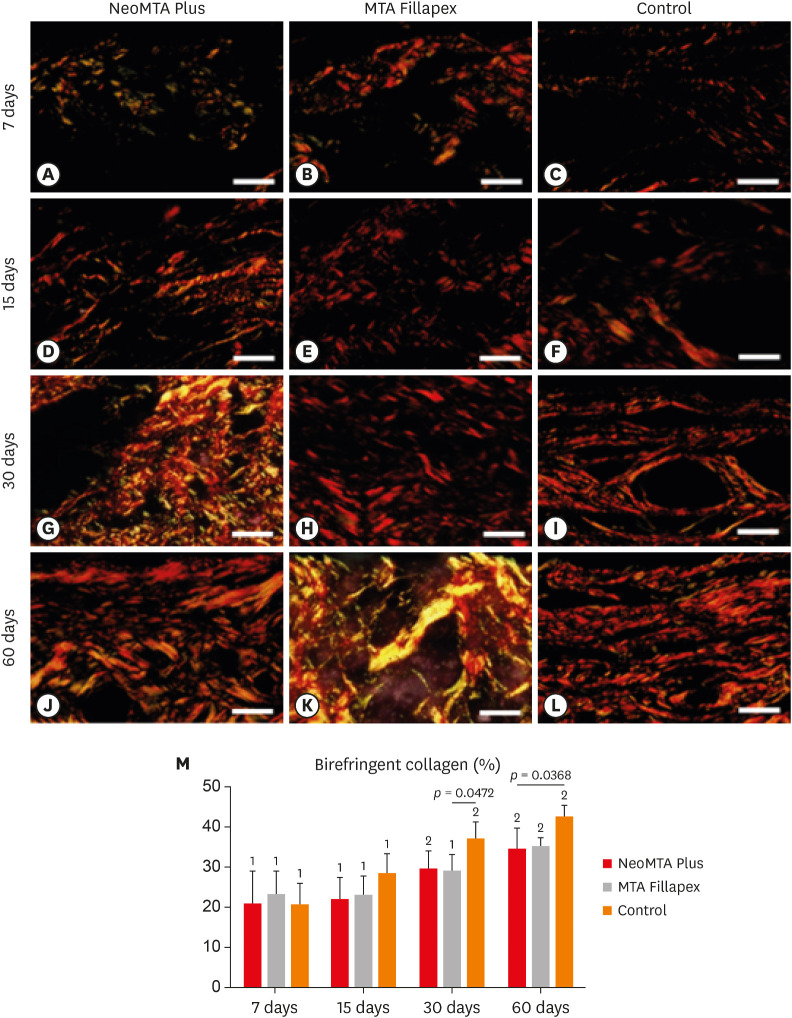
At 7 days, the capsules around NeoMTA Plus and MTA Fillapex had more ICs and IL-6-immunostained cells than the CG. However, at 60 days, there was no significant difference in the IC number between NeoMTA Plus and the CG (p = 0.1137) or the MTA Fillapex group (p = 0.4062), although a greater number of IL-6-immunostained cells was observed in the MTA Fillapex group (p = 0.0353). From 7 to 60 days, the capsule thickness of the NeoMTA Plus and MTA Fillapex specimens significantly decreased, concomitantly with an increase in the collagen content. The capsules around root canal sealers showed positivity to the von Kossa stain and birefringent structures.
Conclusions
The NeoMTA Plus root canal sealer is biocompatible and exhibits bioactive potential.
Keyword
Figure
Reference
-
1. Tomás-Catalá CJ, Collado-González M, García-Bernal D, Oñate-Sánchez RE, Forner L, Llena C, Lozano A, Moraleda JM, Rodríguez-Lozano FJ. Biocompatibility of new pulp-capping materials NeoMTA Plus, MTA Repair HP, and biodentine on human dental pulp stem cells. J Endod. 2018; 44:126–132. PMID: 29079052.
Article2. Cintra LTA, Benetti F, de Azevedo Queiroz ÍO, de Araújo Lopes JM, Penha de Oliveira SH, Sivieri Araújo G, Gomes-Filho JE. Cytotoxicity, biocompatibility, and biomineralization of the new high-plasticity MTA Material. J Endod. 2017; 43:774–778. PMID: 28320539.
Article3. Mondelli JAS, Hoshino RA, Weckwerth PH, Cerri PS, Leonardo RT, Guerreiro-Tanomaru JM, Tanomaru-Filho M, da Silva GF. Biocompatibility of mineral trioxide aggregate flow and biodentine. Int Endod J. 2019; 52:193–200. PMID: 30035812.
Article4. Parirokh M, Torabinejad M, Dummer PMH. Mineral trioxide aggregate and other bioactive endodontic cements: an updated overview - Part I: Vital pulp therapy. Int Endod J. 2018; 51:177–205. PMID: 28836288.
Article5. Siboni F, Taddei P, Prati C, Gandolfi MG. Properties of NeoMTA Plus and MTA Plus cements for endodontics. Int Endod J. 2017; 50(Supplement 2):e83–e94. PMID: 28452115.6. Slompo C, Peres-Buzalaf C, Gasque KC, Damante CA, Ordinola-Zapata R, Duarte MA, de Oliveira RC. Experimental calcium silicate-based cement with and without zirconium oxide modulates fibroblasts viability. Braz Dent J. 2015; 26:587–591. PMID: 26963200.
Article7. Bozeman TB, Lemon RR, Eleazer PD. Elemental analysis of crystal precipitate from gray and white MTA. J Endod. 2006; 32:425–428. PMID: 16631841.
Article8. Torabinejad M, Parirokh M. Mineral trioxide aggregate: a comprehensive literature review - Part II: Leakage and biocompatibility investigations. J Endod. 2010; 36:190–202. PMID: 20113774.
Article9. McMichael GE, Primus CM, Opperman LA. Dentinal tubule penetration of tricalcium silicate sealers. J Endod. 2016; 42:632–636. PMID: 26898564.
Article10. Siboni F, Taddei P, Zamparini F, Prati C, Gandolfi MG. Properties of BioRoot RCS, a tricalcium silicate endodontic sealer modified with povidone and polycarboxylate. Int Endod J. 2017; 50(Supplement 2):e120–e136. PMID: 28881478.
Article11. Quintana RM, Jardine AP, Grechi TR, Grazziotin-Soares R, Ardenghi DM, Scarparo RK, Grecca FS, Kopper PMP. Bone tissue reaction, setting time, solubility, and pH of root repair materials. Clin Oral Investig. 2019; 23:1359–1366.
Article12. Tanomaru-Filho M, Andrade AS, Rodrigues EM, Viola KS, Faria G, Camilleri J, Guerreiro-Tanomaru JM. Biocompatibility and mineralized nodule formation of NeoMTA Plus and an experimental tricalcium silicate cement containing tantalum oxide. Int Endod J. 2017; 50(Supplement 2):e31–e39. PMID: 28390072.13. Prüllage RK, Urban K, Schäfer E, Dammaschke T. Material properties of a tricalcium silicate-containing, a mineral trioxide aggregate-containing, and an epoxy resin-based root canal sealer. J Endod. 2016; 42:1784–1788. PMID: 27769676.
Article14. Collado-González M, García-Bernal D, Oñate-Sánchez RE, Ortolani-Seltenerich PS, Lozano A, Forner L, Llena C, Rodríguez-Lozano FJ. Biocompatibility of three new calcium silicate-based endodontic sealers on human periodontal ligament stem cells. Int Endod J. 2017; 50:875–884. PMID: 27666949.
Article15. Camilleri J, Montesin FE, Papaioannou S, McDonald F, Pitt Ford TR. Biocompatibility of two commercial forms of mineral trioxide aggregate. Int Endod J. 2004; 37:699–704. PMID: 15347295.
Article16. Tanomaru-Filho M, Cristine Prado M, Torres FFE, Viapiana R, Pivoto-João MMB, Guerreiro-Tanomaru JM. Physicochemical properties and bioactive potential of a new epoxy resin-based root canal sealer. Braz Dent J. 2019; 30:563–568. PMID: 31800750.
Article17. Delfino MM, Guerreiro-Tanomaru JM, Tanomaru-Filho M, Sasso-Cerri E, Cerri PS. Immunoinflammatory response and bioactive potential of GuttaFlow bioseal and MTA Fillapex in the rat subcutaneous tissue. Sci Rep. 2020; 10:7173. PMID: 32346066.
Article18. Silva GF, Tanomaru-Filho M, Bernardi MI, Guerreiro-Tanomaru JM, Cerri PS. Niobium pentoxide as radiopacifying agent of calcium silicate-based material: evaluation of physicochemical and biological properties. Clin Oral Investig. 2015; 19:2015–2025.
Article19. da Fonseca TS, da Silva GF, Tanomaru-Filho M, Sasso-Cerri E, Guerreiro-Tanomaru JM, Cerri PS. In vivo evaluation of the inflammatory response and IL-6 immunoexpression promoted by biodentine and MTA Angelus. Int Endod J. 2016; 49:145–153. PMID: 25644518.
Article20. Saraiva JA, da Fonseca TS, da Silva GF, Sasso-Cerri E, Guerreiro-Tanomaru JM, Tanomaru-Filho M, Cerri PS. Reduced interleukin-6 immunoexpression and birefringent collagen formation indicate that MTA Plus and MTA Fillapex are biocompatible. Biomed Mater. 2018; 13:035002. PMID: 29242419.
Article21. de Pizzol Júnior JP, Sasso-Cerri E, Cerri PS. Matrix metalloproteinase-1 and acid phosphatase in the degradation of the lamina propria of eruptive pathway of rat molars. Cells. 2018; 7:e206. PMID: 30423799.
Article22. Silva GF, Guerreiro-Tanomaru JM, da Fonseca TS, Bernardi MIB, Sasso-Cerri E, Tanomaru-Filho M, Cerri PS. Zirconium oxide and niobium oxide used as radiopacifiers in a calcium silicate-based material stimulate fibroblast proliferation and collagen formation. Int Endod J. 2017; 50(Supplement 2):e95–e108. PMID: 28470859.
Article23. Hoshino RA, Silva GF, Delfino MM, Guerreiro-Tanomaru JM, Tanomaru-Filho M, Sasso-Cerri E, Filho IB, Cerri PS. Physical properties, antimicrobial activity and in vivo tissue response to Apexit Plus. Materials (Basel). 2020; 13:E1171. PMID: 32151089.24. Holland R, de Souza V, Nery MJ, Otoboni Filho JA, Bernabé PF, Dezan Júnior E. Reaction of rat connective tissue to implanted dentin tubes filled with mineral trioxide aggregate or calcium hydroxide. J Endod. 1999; 25:161–166. PMID: 10321179.
Article25. Gomes-Filho JE, Watanabe S, Bernabé PF, de Moraes Costa MT. A mineral trioxide aggregate sealer stimulated mineralization. J Endod. 2009; 35:256–260. PMID: 19166785.
Article26. Arias-Moliz MT, Camilleri J. The effect of the final irrigant on the antimicrobial activity of root canal sealers. J Dent. 2016; 52:30–36. PMID: 27377571.
Article27. Li Y, Chi L, Stechschulte DJ, Dileepan KN. Histamine-induced production of interleukin-6 and interleukin-8 by human coronary artery endothelial cells is enhanced by endotoxin and tumor necrosis factor-alpha. Microvasc Res. 2001; 61:253–262. PMID: 11336536.
Article28. de Oliveira PA, de Pizzol-Júnior JP, Longhini R, Sasso-Cerri E, Cerri PS. Cimetidine reduces interleukin-6, matrix metalloproteinases-1 and -9 immunoexpression in the gingival mucosa of rat molars with induced periodontal disease. J Periodontol. 2017; 88:100–111. PMID: 27587368.
Article29. Kang S, Tanaka T, Narazaki M, Kishimoto T. Targeting interleukin-6 signaling in clinic. Immunity. 2019; 50:1007–1023. PMID: 30995492.
Article30. Hashizume M, Mihara M. The roles of interleukin-6 in the pathogenesis of rheumatoid arthritis. Arthritis (Egypt). 2011; 2011:765624.
Article31. Silva EJ, Rosa TP, Herrera DR, Jacinto RC, Gomes BP, Zaia AA. Evaluation of cytotoxicity and physicochemical properties of calcium silicate-based endodontic sealer MTA Fillapex. J Endod. 2013; 39:274–277. PMID: 23321245.
Article32. James SK, Oldgren J, Lindbäck J, Johnston N, Siegbahn A, Wallentin L. An acute inflammatory reaction induced by myocardial damage is superimposed on a chronic inflammation in unstable coronary artery disease. Am Heart J. 2005; 149:619–626. PMID: 15990743.
Article33. Narazaki M, Tanaka T, Kishimoto T. The role and therapeutic targeting of IL-6 in rheumatoid arthritis. Expert Rev Clin Immunol. 2017; 13:535–551. PMID: 28494214.
Article34. Noh MK, Jung M, Kim SH, Lee SR, Park KH, Kim DH, Kim HH, Park YG. Assessment of IL-6, IL-8 and TNF-α levels in the gingival tissue of patients with periodontitis. Exp Ther Med. 2013; 6:847–851. PMID: 24137277.
Article35. Poggio C, Riva P, Chiesa M, Colombo M, Pietrocola G. Comparative cytotoxicity evaluation of eight root canal sealers. J Clin Exp Dent. 2017; 9:e574–e578. PMID: 28469826.
Article36. Vitti RP, Prati C, Silva EJ, Sinhoreti MA, Zanchi CH, de Souza e Silva MG, Ogliari FA, Piva E, Gandolfi MG. Physical properties of MTA Fillapex sealer. J Endod. 2013; 39:915–918. PMID: 23791263.
Article37. Yaltirik M, Ozbas H, Bilgic B, Issever H. Reactions of connective tissue to mineral trioxide aggregate and amalgam. J Endod. 2004; 30:95–99. PMID: 14977305.
Article38. Cintra LT, Ribeiro TA, Gomes-Filho JE, Bernabé PF, Watanabe S, Facundo AC, Samuel RO, Dezan-Júnior E. Biocompatibility and biomineralization assessment of a new root canal sealer and root-end filling material. Dent Traumatol. 2013; 29:145–150. PMID: 22510310.
Article39. Bueno CR, Valentim D, Marques VA, Gomes-Filho JE, Cintra LT, Jacinto RC, Dezan-Junior E. Biocompatibility and biomineralization assessment of bioceramic-, epoxy-, and calcium hydroxide-based sealers. Braz Oral Res. 2016; 30:e81.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Biocompatibility and Bioactivity of Four Different Root Canal Sealers in Osteoblastic Cell Line MC3T3-El
- Flow characteristics and alkalinity of novel bioceramic root canal sealers
- Retreatability of two endodontic sealers, EndoSequence BC Sealer and AH Plus: a micro-computed tomographic comparison
- Nanoleakage of apical sealing using a calcium silicate-based sealer according to canal drying methods
- Bacterial leakage and micro-computed tomography evaluation in round-shaped canals obturated with bioceramic cone and sealer using matched single cone technique