J Korean Med Sci.
2023 Nov;38(45):e381. 10.3346/jkms.2023.38.e381.
Promoter-Specific Variants in NeuroD1 and H3K4me3 Coincident Regions and Clinical Outcomes of Small Cell Lung Cancer
- Affiliations
-
- 1Department of Internal Medicine, School of Medicine, Kyungpook National University, Daegu, Korea
- 2Department of Biochemistry and Cell Biology, School of Medicine, Kyungpook National University, Daegu, Korea
- 3BK21 Plus KNU Biomedical Convergence Program, Department of Biomedical Science, Kyungpook National University, Daegu, Korea
- 4Cell and Matrix Research Institute, School of Medicine, Kyungpook National University, Daegu, Korea
- 5Medical Research Collaboration Center in Kyungpook National University Hospital and School of Medicine, Kyungpook National University, Daegu, Korea
- KMID: 2547983
- DOI: http://doi.org/10.3346/jkms.2023.38.e381
Abstract
- Background
Neurogenic differentiation 1 (NeuroD1) is a representative small cell lung cancer (SCLC) transcription regulator involved in the carcinogenesis and behavior of SCLC. Histone modifications play an important role in transcription, and H3 lysine 4 trimethylation (H3K4me3) is primarily associated with promoter regions.
Methods
We investigated the association between single nucleotide polymorphisms (SNPs) in NeuroD1 and H3K4me3 coincident regions, selected using ChIP sequencing (ChIP-seq), and the clinical outcomes of 261 patients with SCLC.
Results
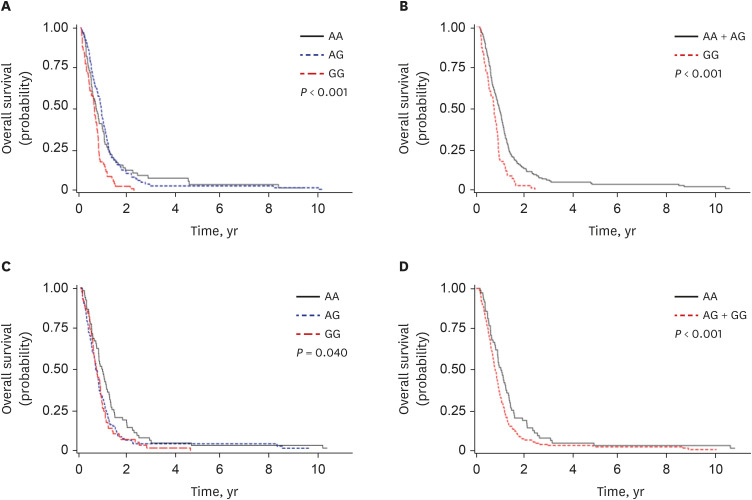
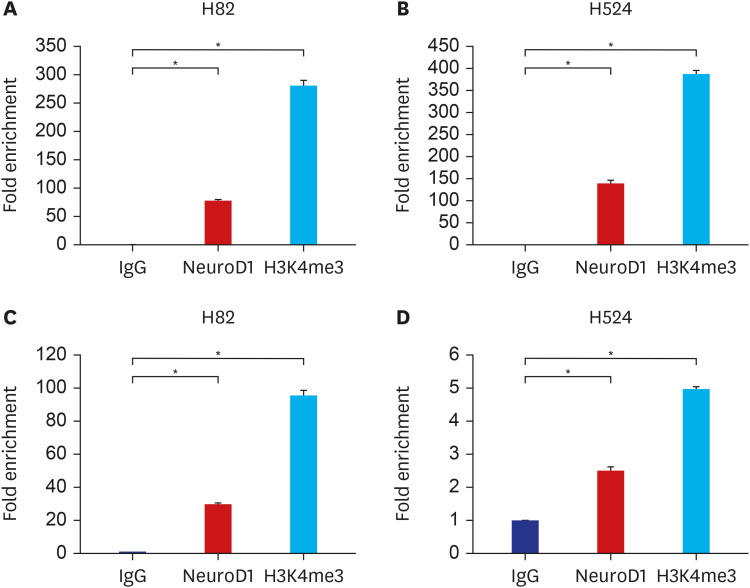
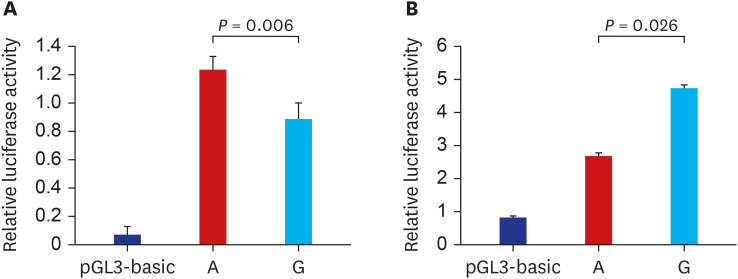
Among 230 SNPs, two were significantly associated with both the chemotherapy response and overall survival (OS) of patients with SCLC. RNF145 rs2043268A>G was associated with worse chemotherapy response and OS (under a recessive model, adjusted odds ratio [aOR], 0.50, 95% confidence interval [CI], 0.26–0.94, P = 0.031, and adjusted hazard ratio [aHR], 1.88, 95% CI, 1.38–2.57, P < 0.001). CINP rs762105A>G was also associated with worse chemotherapy response and OS (under a dominant model, aOR, 0.47, 95% CI, 0.23–0.99, P = 0.046, and aHR, 2.03, 95% CI, 1.47–2.82, P < 0.001). ChIP–quantitative polymerase chain reaction and luciferase assay confirmed that the two SNPs were located in the active promoter regions and influenced the promoter activity of each gene.
Conclusion
To summarize, among SNPs selected using ChIP-seq in promoter regions with high peaks in both NeuroD1 and H3K4me3, RNF145 rs2043268A>G and CINP rs762105A>G were associated with clinical outcomes in patients with SCLC and also affected the promoter activity of each gene.
Keyword
Figure
Reference
-
1. Son M, Yun JW. Cancer mortality projections in Korea up to 2032. J Korean Med Sci. 2016; 31(6):892–901. PMID: 27247498.
Article2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021; 71(1):7–33. PMID: 33433946.
Article3. Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018; 379(23):2220–2229. PMID: 30280641.
Article4. Goldman JW, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2021; 22(1):51–65. PMID: 33285097.5. Stadler ZK, Thom P, Robson ME, Weitzel JN, Kauff ND, Hurley KE, et al. Genome-wide association studies of cancer. J Clin Oncol. 2010; 28(27):4255–4267. PMID: 20585100.
Article6. Liu C, Cui H, Gu D, Zhang M, Fang Y, Chen S, et al. Genetic polymorphisms and lung cancer risk: evidence from meta-analyses and genome-wide association studies. Lung Cancer. 2017; 113:18–29. PMID: 29110844.
Article7. He Y, Liu H, Chen Q, Shao Y, Luo S. Relationships between SNPs and prognosis of breast cancer and pathogenic mechanism. Mol Genet Genomic Med. 2019; 7(9):e871. PMID: 31317673.
Article8. Andrew AS, Gui J, Sanderson AC, Mason RA, Morlock EV, Schned AR, et al. Bladder cancer SNP panel predicts susceptibility and survival. Hum Genet. 2009; 125(5-6):527–539. PMID: 19252927.
Article9. Laytragoon-Lewin N, Cederblad L, Andersson BÅ, Olin M, Nilsson M, Rutqvist LE, et al. Single-nucleotide polymorphisms and cancer risk, tumor recurrence, or survival of head and neck cancer patients. Oncology. 2017; 92(3):161–169. PMID: 27997918.
Article10. Baine MK, Hsieh MS, Lai WV, Egger JV, Jungbluth AA, Daneshbod Y, et al. SCLC subtypes defined by ASCL1, NEUROD1, POU2F3, and YAP1: a comprehensive immunohistochemical and histopathologic characterization. J Thorac Oncol. 2020; 15(12):1823–1835. PMID: 33011388.
Article11. Ikematsu Y, Tanaka K, Toyokawa G, Ijichi K, Ando N, Yoneshima Y, et al. NEUROD1 is highly expressed in extensive-disease small cell lung cancer and promotes tumor cell migration. Lung Cancer. 2020; 146:97–104. PMID: 32526603.
Article12. Kim JH, Lee SY, Choi JE, Do SK, Lee JH, Hong MJ, et al. Polymorphism in ASCL1 target gene DDC is associated with clinical outcomes of small cell lung cancer patients. Thorac Cancer. 2020; 11(1):19–28. PMID: 31691490.
Article13. Lee S, Yoo SS, Choi JE, Hong MJ, Do SK, Lee JH, et al. Genetic variants of NEUROD1 target genes are associated with clinical outcomes of small-cell lung cancer patients. Thorac Cancer. 2023; 14(13):1145–1152. PMID: 36935366.
Article14. Guenther MG, Levine SS, Boyer LA, Jaenisch R, Young RA. A chromatin landmark and transcription initiation at most promoters in human cells. Cell. 2007; 130(1):77–88. PMID: 17632057.
Article15. Grubert F, Zaugg JB, Kasowski M, Ursu O, Spacek DV, Martin AR, et al. Genetic control of chromatin states in humans involves local and distal chromosomal interactions. Cell. 2015; 162(5):1051–1065. PMID: 26300125.
Article16. Ernst J, Kheradpour P, Mikkelsen TS, Shoresh N, Ward LD, Epstein CB, et al. Mapping and analysis of chromatin state dynamics in nine human cell types. Nature. 2011; 473(7345):43–49. PMID: 21441907.
Article17. Kang HG, Lee YH, Lee SY, Choi JE, Do SK, Hong MJ, et al. Genetic variants in histone modification regions are associated with the prognosis of lung adenocarcinoma. Sci Rep. 2021; 11(1):21520. PMID: 34728688.
Article18. Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009; 10(3):R25. PMID: 19261174.
Article19. Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009; 25(16):2078–2079. PMID: 19505943.
Article20. Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 2008; 9(9):R137. PMID: 18798982.
Article21. Yu G, Wang LG, He QY. ChIPseeker: an R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics. 2015; 31(14):2382–2383. PMID: 25765347.
Article22. Van der Auwera GA, Carneiro MO, Hartl C, Poplin R, Angel GD, Levy-Moonshine A, et al. From FastQ data to high confidence variant calls: the Genome Analysis Toolkit best practices pipeline. Curr Protoc Bioinformatics. 2013; 43(1110):11.10.1–33.23. DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011; 43(5):491–498. PMID: 21478889.
Article24. McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010; 20(9):1297–1303. PMID: 20644199.
Article25. Mukhopadhyay A, Deplancke B, Walhout AJ, Tissenbaum HA. Chromatin immunoprecipitation (ChIP) coupled to detection by quantitative real-time PCR to study transcription factor binding to DNA in Caenorhabditis elegans . Nat Protoc. 2008; 3(4):698–709. PMID: 18388953.
Article26. Grishina I, Lattes B. A novel Cdk2 interactor is phosphorylated by Cdc7 and associates with components of the replication complexes. Cell Cycle. 2005; 4(8):1120–1126. PMID: 16082200.
Article27. Lovejoy CA, Xu X, Bansbach CE, Glick GG, Zhao R, Ye F, et al. Functional genomic screens identify CINP as a genome maintenance protein. Proc Natl Acad Sci U S A. 2009; 106(46):19304–19309. PMID: 19889979.
Article28. Wu Q, Fu C, Li M, Li J, Li Z, Qi L, et al. CINP is a novel cofactor of KLF5 required for its role in the promotion of cell proliferation, survival and tumor growth. Int J Cancer. 2019; 144(3):582–594. PMID: 30289973.
Article29. Cook EC, Nelson JK, Sorrentino V, Koenis D, Moeton M, Scheij S, et al. Identification of the ER-resident E3 ubiquitin ligase RNF145 as a novel LXR-regulated gene. PLoS One. 2017; 12(2):e0172721. PMID: 28231341.
Article30. Jiang LY, Jiang W, Tian N, Xiong YN, Liu J, Wei J, et al. Ring finger protein 145 (RNF145) is a ubiquitin ligase for sterol-induced degradation of HMG-CoA reductase. J Biol Chem. 2018; 293(11):4047–4055. PMID: 29374057.
Article31. McDonnell DP, Park S, Goulet MT, Jasper J, Wardell SE, Chang CY, et al. Obesity, cholesterol metabolism, and breast cancer pathogenesis. Cancer Res. 2014; 74(18):4976–4982. PMID: 25060521.
Article32. Lin CY, Huo C, Kuo LK, Hiipakka RA, Jones RB, Lin HP, et al. Cholestane-3β, 5α, 6β-triol suppresses proliferation, migration, and invasion of human prostate cancer cells. PLoS One. 2013; 8(6):e65734. PMID: 23785446.
Article33. Mei Z, Liang M, Li L, Zhang Y, Wang Q, Yang W. Effects of statins on cancer mortality and progression: a systematic review and meta-analysis of 95 cohorts including 1,111,407 individuals. Int J Cancer. 2017; 140(5):1068–1081. PMID: 27859151.
Article34. Manthravadi S, Shrestha A, Madhusudhana S. Impact of statin use on cancer recurrence and mortality in breast cancer: a systematic review and meta-analysis. Int J Cancer. 2016; 139(6):1281–1288. PMID: 27176735.
Article35. Hwang KE, Na KS, Park DS, Choi KH, Kim BR, Shim H, et al. Apoptotic induction by simvastatin in human lung cancer A549 cells via Akt signaling dependent down-regulation of survivin. Invest New Drugs. 2011; 29(5):945–952. PMID: 20464445.
Article36. Chen J, Liu B, Yuan J, Yang J, Zhang J, An Y, et al. Atorvastatin reduces vascular endothelial growth factor (VEGF) expression in human non-small cell lung carcinomas (NSCLCs) via inhibition of reactive oxygen species (ROS) production. Mol Oncol. 2012; 6(1):62–72. PMID: 22153388.
Article37. Liu H, Wang Z, Li Y, Li W, Chen Y. Simvastatin prevents proliferation and bone metastases of lung adenocarcinoma in vitro and in vivo. Neoplasma. 2013; 60(3):240–246. PMID: 23373992.
Article38. Chen Y, Li X, Zhang R, Xia Y, Shao Z, Mei Z. Effects of statin exposure and lung cancer survival: a meta-analysis of observational studies. Pharmacol Res. 2019; 141:357–365. PMID: 30641276.
Article39. Xia DK, Hu ZG, Tian YF, Zeng FJ. Statin use and prognosis of lung cancer: a systematic review and meta-analysis of observational studies and randomized controlled trials. Drug Des Devel Ther. 2019; 13:405–422.40. Rong L, Chen B, Liu K, Liu B, He X, Liu J, et al. CircZDBF2 up-regulates RNF145 by ceRNA model and recruits CEBPB to accelerate oral squamous cell carcinoma progression via NFκB signaling pathway. J Transl Med. 2022; 20(1):148. PMID: 35365168.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Analysis of H3K4me3-ChIP-Seq and RNA-Seq data to understand the putative role of miRNAs and their target genes in breast cancer cell lines
- AKAP12alpha is Associated with Promoter Methylation in Lung Cancer
- Functional annotation of lung cancer‒associated genetic variants by cell type‒specific epigenome and long-range chromatin interactome
- Clinical Significance of Aberrant Wnt7a Promoter Methylation in Human Non-Small Cell Lung Cancer in Koreans
- Detection of Serum Free DNA Hypermethylation in Surgically Resected Adenocarcinoma of the Lung